Drug Price Trends for OTEZLA
✉ Email this page to a colleague
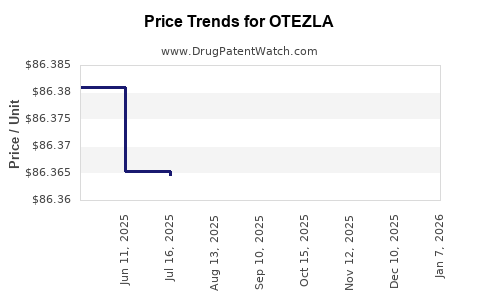
Average Pharmacy Cost for OTEZLA
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| OTEZLA 28 DAY STARTER PACK | 55513-0369-55 | 85.19422 | EACH | 2024-04-17 |
| OTEZLA 30 MG TABLET | 55513-0137-60 | 78.12284 | EACH | 2024-01-05 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for OTEZLA
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| OTEZLA 30MG TAB | Amgen USA, Inc. | 55513-0137-60 | 60 | 3207.49 | 53.45817 | EACH | 2024-01-01 - 2026-01-31 | Big4 |
| OTEZLA 30MG TAB | Amgen USA, Inc. | 55513-0137-60 | 60 | 3312.83 | 55.21383 | EACH | 2024-01-01 - 2026-01-31 | FSS |
| OTEZLA STARTER PACK 10/20/30MG 55 COUNT BOTTL | Amgen USA, Inc. | 55513-0369-55 | 55 | 2393.09 | 43.51073 | EACH | 2021-02-01 - 2026-01-31 | Big4 |
| OTEZLA STARTER PACK 10/20/30MG 55 COUNT BOTTL | Amgen USA, Inc. | 55513-0369-55 | 55 | 2815.60 | 51.19273 | EACH | 2021-02-01 - 2026-01-31 | FSS |
| OTEZLA STARTER PACK 10/20/30MG 55 COUNT BOTTL | Amgen USA, Inc. | 55513-0369-55 | 55 | 2562.12 | 46.58400 | EACH | 2022-01-01 - 2026-01-31 | Big4 |
| OTEZLA 30MG TAB | Amgen USA, Inc. | 55513-0137-60 | 60 | 2472.38 | 41.20633 | EACH | 2021-02-01 - 2026-01-31 | Big4 |
| OTEZLA STARTER PACK 10/20/30MG 55 COUNT BOTTL | Amgen USA, Inc. | 55513-0369-55 | 55 | 2967.36 | 53.95200 | EACH | 2022-01-01 - 2026-01-31 | FSS |
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |


