Share This Page
Drug Price Trends for OMEPRAZOLE MAG DR
✉ Email this page to a colleague
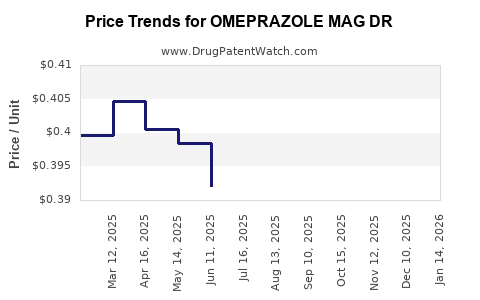
Average Pharmacy Cost for OMEPRAZOLE MAG DR
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| OMEPRAZOLE MAG DR 20 MG CAP | 83324-0117-14 | 0.39835 | EACH | 2025-12-17 |
| OMEPRAZOLE MAG DR 20 MG TABLET | 00536-1322-13 | 0.37441 | EACH | 2025-12-17 |
| OMEPRAZOLE MAG DR 20 MG TABLET | 00536-1322-71 | 0.37441 | EACH | 2025-12-17 |
| OMEPRAZOLE MAG DR 20 MG CAP | 83324-0117-42 | 0.39835 | EACH | 2025-12-17 |
| OMEPRAZOLE MAG DR 20.6 MG CAP | 55111-0397-52 | 0.39835 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for OMEPRAZOLE MAG DR
Overview of OMEPRAZOLE MAG DR
OMEPRAZOLE MAG DR is a delayed-release formulation of omeprazole, a widely used proton pump inhibitor (PPI) indicated for conditions such as gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger-Ellison syndrome. Its efficacy in reducing gastric acid secretion has established it as a staple in gastroenterology therapeutics.
The drug's key differentiation lies in its magnesium-based, delayed-release formulation, offering sustained acid suppression with improved safety features. Manufacturing complexity, patent landscape, and clinical efficacy contribute to its positioning in the market.
Global Market Landscape
Market Size and Growth Potential
The global PPI market was valued at approximately $12 billion in 2022, with omeprazole representing a significant share. The increasing prevalence of acid-related disorders—termed a persistent global health concern—underpins the sustained demand (1).
In particular, the delayed-release formulations like OMEPRAZOLE MAG DR benefit from rising prescription rates due to their improved pharmacokinetics and patient compliance. The Asia-Pacific region exhibits high growth potential driven by expanding healthcare access and lifestyle changes, with projections indicating a CAGR of over 5% through 2030 (2).
Key Regional Markets
- North America: Dominates with over 40% of the global market, led by high prescription rates, advanced healthcare infrastructure, and a large aging population.
- Europe: The second-largest market, with mature healthcare systems, stringent regulatory standards, and high prevalence of GERD.
- Asia-Pacific: Rapidly expanding market, with increasing adoption of OTC formulations and rising awareness, forecasted to outpace Western markets in growth.
Competitive Landscape
Major players include AstraZeneca (Nexium), Pfizer (Prevacid), and proprietary formulations. Generic manufacturers, capitalizing on patent expirations, have considerably increased price competition.
OMEPRAZOLE MAG DR's entry is further influenced by patent statuses, regulatory approvals, and clinical positioning. The availability of lower-cost generics underpins price sensitivity, especially in emerging markets.
Regulatory and Patent Dynamics
The patent landscape for omeprazole mainly expired worldwide by the late 2010s, opening avenues for generics. However, formulation-specific patents (such as for delayed-release magnesium formulations) may still afford exclusivity periods, delaying generic competition in certain jurisdictions.
Regulatory hurdles revolve around demonstrating bioequivalence and safety. Ongoing patents and clinical data influence market entry timelines and pricing strategies.
Pricing Dynamics
Current Pricing Trends
- Brand-Name OMEPRAZOLE MAG DR: Premium pricing persists in markets with patent protection and advanced formulations.
- Generics and Bioequivalents: Significantly lower cost, often 20-50% cheaper than the branded drug, influencing prescribing decisions and consumer choices.
In North America, the retail price for a 30-day supply of OMEPRAZOLE MAG DR can range from $100 to $150, while generics typically cost between $25 and $50 (3).
In emerging markets, prices are substantially lower due to regulatory price controls, generic availability, and purchasing power parity.
Factors Influencing Price
- Regulatory Approval: Extended approval periods and reimbursement policies impact pricing.
- Manufacturing Costs: Complexity of delayed-release magnesium formulations causes higher costs relative to conventional PPIs.
- Market Competition: The rising presence of generics and alternate therapies exerts downward pressure.
- Reimbursement Policies: Insurance coverage and government healthcare schemes significantly influence consumer out-of-pocket costs.
Projected Price Trends (2023-2030)
- Short-term (2023-2025): Prices in mature markets are expected to decline gradually due to increasing generic penetration, with brand-name prices stabilizing or slightly decreasing.
- Medium to Long-term (2026-2030): Price erosion is anticipated as patent exclusivity diminishes further, potentially leading to a 30-50% reduction in average drug prices, in line with historical trends in the PPI class.
- Market Entry of Biosimilars and Extended-Release Variants: These developments could further exert downward pressure on prices.
In emerging regions, prices will likely remain stable or even rise modestly due to increasing demand and limited local manufacturing capabilities.
Market Drivers and Barriers
Drivers
- Rising prevalence of acid-related disorders globally.
- Growing aging population requiring chronic PPI therapy.
- Increasing awareness and acceptance of delayed-release formulations.
- Expansion of OTC availability in select markets.
Barriers
- Stringent regulatory approval processes.
- Patent litigation delays for generic entrants.
- Market saturation in developed countries.
- Pricing caps in healthcare systems.
Key Market Trends
- Expansion of OTC availability for PPIs, including delayed-release formulations.
- Growing preference for combination therapies, potentially affecting standalone drug sales.
- Increasing focus on drug safety, prompting formulation innovations.
- Digital health innovations facilitating patient adherence and monitoring.
Conclusion and Future Outlook
The market for OMEPRAZOLE MAG DR is poised for steady growth driven by global trends in acid-related disease prevalence. Patent expirations and the subsequent influx of generics threaten to compress prices, especially in mature markets. Conversely, technological advances and regulatory environments will influence future pricing strategies.
In the next 5-7 years, a continued downward trajectory in branded drug prices is expected, with additional market entries from biosimilars and formulations. Companies should innovate to sustain profitability, focusing on differentiating features such as improved safety profiles, topical formulations, or combination therapies.
Key Takeaways
- Market Expansion: Growing global burden of GERD and related disorders underpins increasing demand for delayed-release omeprazole formulations.
- Price Trends: Anticipate significant generic-driven price reductions, with brand premiums diminishing over time.
- Regional Variations: Developed markets project greater price erosion; emerging markets may maintain higher prices due to limited competition.
- Strategic Positioning: Firms should focus on innovation, patent strategies, and expanding OTC access to sustain market share.
- Regulatory Dynamics: Keeping abreast of patent laws and approval pathways is critical to timing market entry and price optimization.
FAQs
1. How does patent expiration impact the pricing of OMEPRAZOLE MAG DR?
Patent expirations open the market to generic manufacturers, boosting competition and generally leading to significant price reductions. Timely entry of generics can lower prices by up to 50% or more.
2. What factors influence the price of delayed-release magnesium formulations compared to standard PPIs?
Manufacturing complexity, patent protections, regulatory approval processes, and market competition all influence pricing. Delayed-release formulations often command a premium due to advanced technology.
3. How are emerging markets expected to affect the global pricing landscape?
Lower manufacturing costs, regulatory variances, and increased generic availability typically keep prices lower in emerging markets, though local economic factors and healthcare policies can influence affordability.
4. What role does OTC availability play in the future market for OMEPRAZOLE MAG DR?
Expanded OTC access increases consumer choice, promotes self-medication, and can drive volume sales, but may put additional downward pressure on prices and brand loyalty.
5. Which technological or regulatory trends could influence future price projections?
Innovation in drug delivery, biosimilar development, and regulatory incentives for biosimilars or reimbursable formulations are likely to influence future price dynamics.
Sources:
- [MarketWatch, 2022. Global Proton Pump Inhibitors Market Data Report]
- [Frost & Sullivan, 2022. Asia-Pacific Digestive Health Market Analysis]
- [GoodRx, 2023. Prescription Drug Pricing Trends]
More… ↓
