Drug Price Trends for GNP CHILD ALLERGY
✉ Email this page to a colleague
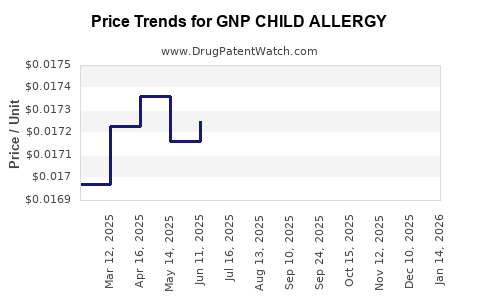
Average Pharmacy Cost for GNP CHILD ALLERGY
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| GNP CHILD ALLERGY 12.5 MG/5 ML | 46122-0674-26 | 0.01650 | ML | 2024-11-20 |
| GNP CHILD ALLERGY 12.5 MG/5 ML | 46122-0674-26 | 0.01762 | ML | 2024-10-23 |
| GNP CHILD ALLERGY 12.5 MG/5 ML | 46122-0674-26 | 0.01686 | ML | 2024-09-18 |
| GNP CHILD ALLERGY 12.5 MG/5 ML | 46122-0674-26 | 0.01624 | ML | 2024-08-21 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |


