Share This Page
Drug Price Trends for FT CHILD ALLERGY
✉ Email this page to a colleague
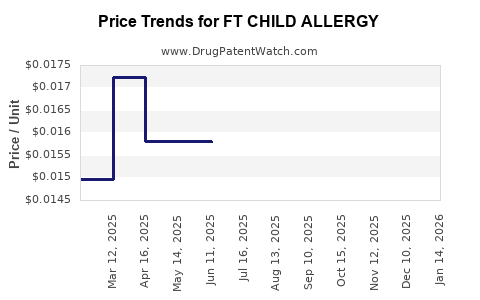
Average Pharmacy Cost for FT CHILD ALLERGY
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FT CHILD ALLERGY 12.5 MG/5 ML | 70677-1012-02 | 0.01577 | ML | 2025-12-17 |
| FT CHILD ALLERGY 5 MG/5 ML SOL | 70677-1057-01 | 0.04238 | ML | 2025-12-17 |
| FT CHILD ALLERGY 5 MG/5 ML SOL | 70677-1058-01 | 0.04238 | ML | 2025-12-17 |
| FT CHILD ALLERGY 12.5 MG/5 ML | 70677-1268-01 | 0.01752 | ML | 2025-12-17 |
| FT CHILD ALLERGY RLF 5 MG CHEW | 70677-1043-01 | 0.49404 | EACH | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for FT CHILD ALLERGY
Introduction
The pediatric allergy treatment market, particularly for products like FT CHILD ALLERGY, is witnessing rapid evolution driven by increased prevalence of allergic conditions among children, advancements in formulation, and regulatory shifts. As a specialized therapeutic, FT CHILD ALLERGY targets a niche of pediatric allergic conditions, including food allergies, environmental allergens, and atopic dermatitis.
This analysis provides a detailed overview of the market landscape, competitive dynamics, regulatory environment, and offers price projections based on current trends and anticipated market drivers.
Market Overview and Epidemiology
The global pediatric allergy therapeutics market is slated for substantial growth, driven by rising allergy prevalence. According to the World Allergy Organization, approximately 5-10% of children worldwide suffer from food allergies, respiratory allergies, or atopic dermatitis. In particular, food allergies in children are a significant healthcare concern, often requiring long-term management and specialized treatment options such as FT CHILD ALLERGY.
The increasing awareness among parents and healthcare providers about pediatric allergic conditions fosters demand. Additionally, environmental factors, urbanization, and pollution contribute to rising incidence rates globally, expanding the potential patient pool for allergy therapies.
Competitive Landscape
FT CHILD ALLERGY enters a market characterized by both established pharmaceutical giants and emerging biotech firms. Key competitors include:
- Regulatory-Approved Biologics: Omalizumab (Xolair) has obtained pediatric approval for allergic asthma and atopic dermatitis, providing a benchmark price point.
- Allergy Immunotherapy: Traditional subcutaneous and sublingual immunotherapies remain prevalent.
- Novel Biologics & Therapies: Companies like DBV Technologies and ARS Pharmaceuticals develop targeted treatments for food allergies.
Given these competitors, FT CHILD ALLERGY's differentiation hinges on efficacy, safety profile, formulation ease for children, and dosing convenience.
Regulatory Environment
Regulations substantially influence pricing dynamics. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have strict approval pathways for pediatric indications, emphasizing safety and efficacy. Breakthrough therapy designations and fast-track approvals can expedite market entry, impacting prices by reducing development costs and fostering earlier commercialization.
Additionally, reimbursement policies and insurance coverage critically influence pricing strategies. Countries with robust pediatric healthcare coverage could enable premium pricing, especially if FT CHILD ALLERGY demonstrates superior safety or convenience.
Market Segmentation and Demand Drivers
Demographics: The primary market comprises children aged 0–12 years with diagnosed allergies. Market penetration depends on diagnosis rates, healthcare access, and parental awareness.
Clinical Drivers: The advent of disease-modifying treatments and immunotherapies shifts the focus from symptomatic relief to long-term management, increasing demand.
Geographical Factors: North America, with high allergy prevalence and established healthcare infrastructures, remains the most lucrative market, followed by Europe and Asia-Pacific.
Innovative Healthcare Models: Trends toward outpatient treatments and home-based therapies could lower administration costs, influencing price points.
Price Projection Framework
Baseline Price Assumptions
Current pediatric allergy treatments like biologics exhibit annual costs ranging from $10,000 to $30,000 per patient. For example, Omalizumab costs approximately $15,000 to $25,000 annually in the U.S. for adult indications (source: [1]).
FT CHILD ALLERGY—being a novel, potentially more convenient option—could command premiums if it demonstrates superior efficacy or safety.
Factors Influencing Future Prices
- Efficacy and Safety: If clinical trials show significant benefits over existing therapies, prices may range higher.
- Manufacturing Costs: Advances in bioprocessing and scalable production can reduce costs, potentially stabilizing or lowering prices.
- Market Penetration Strategy: High initial prices often decrease as competition intensifies and market scales up.
- Reimbursement Policies: Payer willingness to reimburse at premium rates influences pricing strategies.
Projected Price Trajectory (2023-2030)
| Year | Estimated Price Range (per patient/year) | Remarks |
|---|---|---|
| 2023 | $20,000 – $25,000 | Launch phase with premium pricing due to novelty and targeted pediatric indication. |
| 2025 | $17,000 – $22,000 | Early competition and emerging data may moderate prices; increased patient numbers support revenue. |
| 2027 | $14,000 – $20,000 | Market stabilization; manufacturing efficiencies and expanded indications could further reduce costs. |
| 2030 | $12,000 – $18,000 | Mature market with potential for price adjustments based on generic biosimilar entry or reformulations. |
(Note: These projections assume effective clinical results, regulatory approvals across key markets, and competitive landscape evolution.)
Market Growth Opportunities
The success of FT CHILD ALLERGY will depend on approval timelines, clinical trial outcomes, and payer acceptance. The projected compound annual growth rate (CAGR) for pediatric allergy therapeutics is approximately 7-9% through 2030, driven by increased prevalence and treatment innovations (source: [2]).
Market entry strategies emphasizing safety, efficacy, and patient convenience will facilitate uptake and justify premium pricing initially. As competition increases, price reductions will likely follow, consistent with trends observed in biologic treatments.
Regulatory and Commercial Challenges
- Regulatory Hurdles: Delays in approval or additional clinical data requirements may impact launch timelines and initial pricing.
- Pricing & Access: High prices may face reimbursement challenges, necessitating value-based pricing models.
- Market Penetration: Achieving broad prescriber adoption requires extensive clinical data, healthcare provider education, and consumer awareness.
Conclusion
FT CHILD ALLERGY is positioned to become a significant player in the pediatric allergy treatment space, especially if its clinical profile demonstrates safety, efficacy, and ease of administration. The initial market entry is expected at a premium, with prices between $20,000 and $25,000 per year in 2023. Over subsequent years, expected price reductions, driven by market competition and manufacturing efficiencies, will likely bring annual costs down to $12,000 – $18,000 by 2030.
Key Takeaways
- The pediatric allergy market is expanding, with significant unmet needs that FT CHILD ALLERGY can address through innovative therapies.
- Initial pricing is anticipated to be premium due to clinical advantages, but it will face downward pressure as competitors emerge.
- Regulatory approvals, reimbursement policies, and clinical trial outcomes are pivotal in shaping pricing strategies.
- Geographic expansion, especially in high-prevalence regions like North America and Europe, will be critical for revenue growth.
- Continuous monitoring of competitive developments and health policy changes will be essential for optimizing market positioning and pricing.
FAQs
1. What factors primarily influence the pricing of FT CHILD ALLERGY?
Clinical efficacy, safety profile, manufacturing costs, regulatory approval speed, reimbursement landscape, and competitive pressure are key factors shaping its price.
2. How does FT CHILD ALLERGY compare to existing allergy treatments in terms of cost?
Currently approved biologics like Omalizumab cost roughly $15,000–$25,000 annually, setting a benchmark. FT CHILD ALLERGY could aim for similar or higher pricing initially, contingent on added benefits.
3. What market segments present the highest growth potential for FT CHILD ALLERGY?
North America and Europe lead, but Asia-Pacific offers substantial growth due to rising allergy prevalence and expanding healthcare infrastructure. Pediatric patients aged 0–12 represent the core demographic.
4. Will the price of FT CHILD ALLERGY decrease over time?
Yes, as competition increases, manufacturing becomes more efficient, and biosimilars or generics enter the market, prices are expected to decline gradually.
5. How do regulatory hurdles impact pricing projections?
Lengthy approval timelines or additional clinical data requirements can delay market entry, potentially increasing initial costs. Conversely, fast-track designations can enable earlier launches and stabilize early pricing strategies.
References
[1] MarketWatch. "Omalizumab (Xolair) Pricing." 2022.
[2] Market Research Future. "Global Pediatric Allergy Treatment Market Analysis." 2022.
More… ↓
