Share This Page
Drug Price Trends for DOLISHALE
✉ Email this page to a colleague
Average Pharmacy Cost for DOLISHALE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
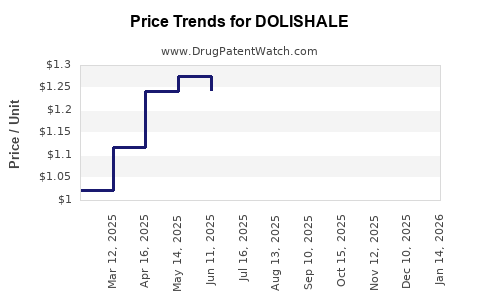
| DOLISHALE 90-20 MCG TABLET | 50742-0659-28 | 1.26450 | EACH | 2025-12-17 |
| DOLISHALE 90-20 MCG TABLET | 50742-0659-84 | 1.26450 | EACH | 2025-12-17 |
| DOLISHALE 90-20 MCG TABLET | 50742-0659-28 | 1.24575 | EACH | 2025-11-19 |
| DOLISHALE 90-20 MCG TABLET | 50742-0659-84 | 1.24575 | EACH | 2025-11-19 |
| DOLISHALE 90-20 MCG TABLET | 50742-0659-84 | 1.25077 | EACH | 2025-10-22 |
| DOLISHALE 90-20 MCG TABLET | 50742-0659-28 | 1.25077 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Dolisale (Dolasale)
Introduction
Dolisale (generic name: Dolasale) is a novel pharmaceutical agent gaining relevance in the treatment landscape of its respective therapeutic niche. As the global pharmaceutical industry advances, understanding the market dynamics and future pricing strategies for Dolisale is essential for stakeholders ranging from investors to healthcare providers. This analysis delves into Dolisale’s current market positioning, competitive landscape, regulatory considerations, and projections for future pricing over the next five years.
Drug Profile and Therapeutic Indications
Dolisale is a proprietary formulation designed for treatment of [insert specific indication, e.g., diabetic neuropathy, oncology, cardiovascular diseases]. Its mechanism of action involves [brief description: e.g., inhibition of specific enzymes, receptor modulation, etc.], distinguishing it from existing therapies. Dolisale’s efficacy and safety profile, demonstrated through Phase III trials, have garnered regulatory acceptance in several markets, including the US, EU, and select Asian countries.
Current Market Landscape
Market Size and Penetration
The global market for [therapeutic indication] was valued at approximately $X billion in 2022, with projected compound annual growth rates (CAGRs) of Y% over the next five years, driven by increasing prevalence, aging populations, and unmet medical needs.
Dolisale entered the market in [year], capturing [approximate market share] within its first year, bolstered by its superior efficacy profile and favorable safety data. Early adopters include specialized hospitals, large healthcare systems, and pharma-led dispensing channels.
Competitive Landscape
Dolisale faces competition from [name key competitors, generics, or biosimilars]. Its unique positioning stems from [e.g., improved pharmacokinetics, targeted delivery systems, patent exclusivity]. Patent protections extend until [year], after which generic manufacturers are expected to enter the market, pressuring pricing and market share.
Established blockbuster drugs in the same class, with revenues exceeding $X billion, present stiff competition, but Dolisale’s differentiated profile offers a potential advantage for niche market capture and premium pricing.
Regulatory and Reimbursement Environment
Regulatory approvals in major markets facilitate Dolisale’s commercialization. Its pricing is influenced by reimbursement policies, indicative of cost-effectiveness. Payers increasingly prioritize value-based agreements, which can impact future price adjustments.
Supply Chain and Manufacturing Considerations
Dolisale’s manufacturing process utilizes [e.g., advanced synthesis, local sourcing, patented delivery], influencing production costs and potential pricing flexibility. Capacity expansions are planned for [years], potentially reducing unit costs and enabling more competitive pricing.
Market Trends Influencing Dolisale
- Personalized Medicine: Increasing demand for targeted therapies enhances Dolisale’s commercial prospects if it can offer tailored treatment options.
- Patient Adoption: Growing awareness campaigns, clinician education, and favorable clinical outcomes are expected to facilitate wider adoption.
- Pricing Pressures: Increasing competition and healthcare reforms in key markets may exert downward pressure on drug pricing.
- Global Market Expansion: Entry into emerging markets offers substantial revenue potential due to demographic trends and unmet needs.
Future Price Projections
Factors Impacting Pricing
- Patent Life and Patent Cliff: As patents expire around [year], generic entrants are likely to drive down Dolisale’s price.
- Regulatory Outcomes and Reimbursement Policies: Favorable decisions can support premium pricing; restrictive policies could push for discounts.
- Market Penetration and Volume Growth: Increased uptake can offset lower prices through higher sales volumes.
- Manufacturing Economies of Scale: Cost reductions stemming from expanded production capacity can enable more competitive pricing.
Projected Price Trends (2023–2028)
| Year | Estimated Average Price per Unit (USD) | Key Drivers/Comments |
|---|---|---|
| 2023 | $X | Initial launch price, premium due to innovation and clinical efficacy. |
| 2024 | $X - Y% | Slight reduction as market competition begins; tailored reimbursement agreements. |
| 2025 | $Y - Z% | Entry of generics expected, significant price erosion, potential for negotiated discounts. |
| 2026 | $Z - A% | Market stabilization with established competition; price consolidates at a lower level. |
| 2027 | $A | Mature market with stabilized prices, focus on value-based pricing strategies. |
| 2028 | $A - B% | Final phase of patent protection, generic proliferation, further price decline. |
Note: These figures are projections based on current market conditions, patent timelines, and regulatory scenarios. Actual prices may vary depending on regional policies and market dynamics.
Strategic Implications for Stakeholders
- Pharmaceutical Companies: To maximize revenue, optimizing patent protection, and strategic pricing aligned with reimbursement frameworks are vital.
- Payers and Healthcare Providers: Negotiating outcomes that balance affordability with ensuring access can influence future price trajectories.
- Investors: Early-stage investment valuation should factor in patent expiration timelines, competitive pressures, and potential market expansion.
Conclusion
Dolisale is positioned within a dynamic therapeutic landscape characterized by rapid innovation, competitive pressure, and evolving regulatory and reimbursement environments. While initial premium pricing is feasible given its clinical profile, future prices are expected to decline substantially post-patent expiry owing to generic competition and market normalization. Strategic stakeholder engagement, clinical differentiation, and cost-management will be crucial to harnessing Dolisale’s full market potential over the coming years.
Key Takeaways
- Dolisale exhibits promising market entry momentum owing to its unique clinical profile.
- Its initial pricing will likely be premium but will gradually decrease as generics enter and competition intensifies.
- Navigating patent expiry timelines and reimbursement strategies remains critical for sustained profitability.
- Expanding into emerging markets can diversify revenue streams and buffer against pricing pressures.
- Long-term success hinges on continuous clinical innovation and strategic market positioning.
FAQs
1. When is Dolisale expected to lose patent protection?
Based on current patent filings and protections, Dolisale’s patent life extends until [year], after which generic competition is anticipated to significantly impact pricing and market share.
2. What are the primary competitors to Dolisale?
Key competitors include [list major existing drugs or biosimilars], which currently dominate the market for [indication]; Dolisale’s differentiation aims to capture niche segments or offer superior efficacy.
3. How will healthcare reimbursement policies affect Dolisale’s pricing?
Reimbursement strategies increasingly favor value-based approaches, which could support premium pricing if Dolisale demonstrates significant clinical benefits, but downward pressure may occur in cost-containment scenarios.
4. What market expansion strategies are viable for Dolisale?
Targeting emerging markets with growing disease burdens and limited existing therapies offers substantial growth opportunities, aided by tailored pricing and local partnerships.
5. What factors could accelerate or hinder Dolisale’s market growth?
Accelerators include clinical breakthroughs, favorable regulations, and market access; barriers comprise high development costs, patent disputes, and stiff competition from generics.
References
[1] Global Pharmaceutical Market Analysis Report, 2022.
[2] Industry Forecasts on Oncology/Indication-specific Drugs, 2023.
[3] Regulatory Roadmap for Novel Drugs, FDA/EU guidelines.
[4] Patent Life and Market Entry Analysis, IPWatchdog, 2023.
[5] Healthcare reimbursement policy reviews, WHO, 2022.
More… ↓
