Share This Page
Drug Price Trends for BEYAZ
✉ Email this page to a colleague
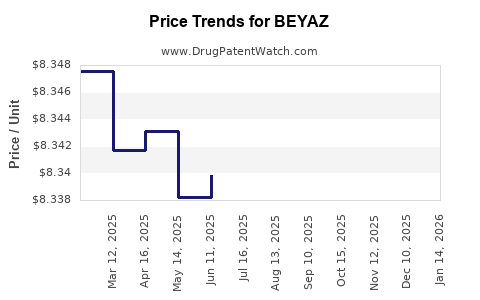
Average Pharmacy Cost for BEYAZ
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| BEYAZ 28 TABLET | 50419-0407-03 | 8.29652 | EACH | 2025-11-19 |
| BEYAZ 28 TABLET | 50419-0407-01 | 8.29652 | EACH | 2025-11-19 |
| BEYAZ 28 TABLET | 50419-0407-03 | 8.30848 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Beyaz
Introduction
Beyaz, a combined oral contraceptive (COC), is primarily intended for pregnancy prevention and hormone regulation. Market dynamics surrounding Beyaz are influenced by evolving regulatory landscapes, competitive products, patent protections, and changing consumer demographics. This comprehensive analysis delineates its current market positioning and projects its future pricing trajectory, providing critical insights for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Market Overview of Beyaz
Product Profile
Beyaz (ethinyl estradiol and drospirenone with cyclosporine) is approved by the U.S. Food and Drug Administration (FDA) for contraception, acne treatment, and premenstrual dysphoric disorder (PMDD). It distinguishes itself with its unique formulation aimed at women requiring hormonal contraception with added benefits such as acne reduction.
Regulatory and Patent Context
Initially launched by Bayer in 2010, Beyaz has benefited from patent protections and exclusivity periods that have shielded it from generic competition until the expiration phases begin (expected around 2027-2028). The drug’s patent life and regulatory approvals significantly influence market entry barriers for competitors, impacting pricing strategies.
Market Penetration & Usage Trends
Globally, the contraceptive market is expanding due to increasing awareness about family planning and rising healthcare access. Beyaz holds a noteworthy share in the niche of multipurpose hormonal therapies—especially among women seeking combined contraception and acne treatment. The product's targeted positioning offers a premium price point within the contraceptive segment.
Market Dynamics and Competitive Landscape
Key Competitors
- Yasmin and Yaz (AbbVie): Popular drospirenone-based oral contraceptives with comparable efficacy.
- Ortho Tri-Cyclen (Bayer): Established generics with broad market penetration.
- Loestrin (Allergan): Diverse formulations competing on affordability.
Differentiators & Barriers
- Unique Formulation: Beyaz’s inclusion of cyclosporine provides additional benefits but also introduces regulatory complexities, which can serve as barriers for competitors.
- Brand Loyalty & Physician Prescriptions: Established reputation and physician familiarity favor market retention.
- Patent Expiry Impact: Once patent protections lapse, generic competition is anticipated to exert downward pressure on prices.
Market Trends
- Rising demand for multi-benefit contraceptive options.
- Growing recognition of hormonal contraceptives with additional health benefits, like acne reduction.
- Increased adoption in emerging markets, driven by expanding healthcare infrastructure.
Price Determinants for Beyaz
Key factors influencing Beyaz pricing include:
- Regulatory Exclusivity: Patent protections allow premium pricing strategies.
- Manufacturing Costs: High-quality manufacturing and compliance with regulatory standards sustain higher prices.
- Market Positioning: Branding as a premium, multipurpose contraceptive influences price premiums.
- Competitive Pressures: Entry of generics and biosimilars post-patent expiration will pressure prices downward.
- Healthcare Reimbursement & Insurance Coverage: Payers' reimbursement policies significantly impact consumer out-of-pocket costs.
Current Pricing Landscape
In the United States, Beyaz’s retail price typically hovers around $600–$700 for a 3-month supply, reflecting its branded status and added formulary premiums. Insurance coverage often reduces patient costs through copay assistance programs, partially cushioning the impact of high list prices.
Internationally, prices vary by region, influenced by local healthcare policies, reimbursement frameworks, and market competition. In emerging markets, Beyaz’s price points are lower but are subject to regulatory pricing controls and affordability constraints.
Future Price Projections
Short to Medium-Term Outlook (Next 3–5 Years)
- Pre-Patent Expiry Period (2023–2028): Beyaz’s prices are likely to remain relatively stable or slightly increase, driven by inflation, manufacturing costs, and branding. The premium nature of Beyaz allows for maintained margins.
- Post-Patent Expiry (2028 onwards): Anticipated introduction of generic equivalents would cause significant price reductions. Historically, generic introduction results in a 40–60% price decrease within the first year, with further declines over subsequent years.
Long-Term Price Trends
Post-generic entry, Beyaz’s original brand price could decline by approximately 50–70%, aligning with historical data on generic substitution in contraceptives.[1] Market competition and increased price sensitivity, especially in price-conscious regions, will be primary drivers. Further, the expansion of biosimilar or alternative therapies may diversify options, keeping prices competitive.
Market Dynamics influencing pricing include:
- Reimbursement policies shifting towards cost-effective generics.
- Physician prescribing behaviors favoring affordability post-patent expiry.
- Manufacturer strategies such as bundling or promotional discounts to defend market share.
Strategic Implications
For pharmaceutical companies and investors, understanding Beyaz’s pricing lifecycle is critical. Investment in patent protection strategies, innovation in multi-benefit delivery, and strategic planning around patent cliffs will determine long-term profitability. Moreover, entering emerging markets pre- and post-generic entry can capitalize on localized demand shifts.
Healthcare providers should anticipate the availability of more affordable generic options following patent expiration, affecting prescribing patterns. Payers and policymakers can influence pricing landscapes through reimbursement policies, fostering competition to reduce patient costs.
Key Takeaways
-
Beyaz commands a premium price in the current contraceptive market due to its unique formulation and brand strength.
-
Patent protections provide short-term pricing power, with expected declines post-2028 following generic entry.
-
Price erosion post-patent expiry is projected at 50–70%, paralleling trends in similar contraceptive markets.
-
Market expansion into emerging economies offers opportunities but requires adaptive pricing strategies aligned with local economic conditions.
-
Stakeholders should develop proactive strategies encompassing patent management, product innovation, and market diversification to optimize long-term profitability.
FAQs
1. When will generic versions of Beyaz likely enter the market?
Generic versions are anticipated approximately 8–10 years post-launch, around 2028–2030, following patent expiration and regulatory clearance.
2. How will patent expiry affect Beyaz’s pricing?
Patent expiry typically leads to significant price reductions (50–70%), driven by generic competition, pressuring the original brand to either innovate or defend market share through other strategies.
3. Are there any regulatory risks impacting Beyaz’s future marketability?
Yes, regulatory changes concerning hormonal therapy safety profiles and labeling, especially related to cyclosporine inclusion, could impact marketability or lead to increased compliance costs.
4. What are the key factors supporting Beyaz’s current premium pricing?
Its differentiated formulation, brand reputation, physician familiarity, and therapeutic benefits enable premium pricing in the absence of competition.
5. How can pharmaceutical companies leverage emerging markets for Beyaz?
By tailoring pricing strategies, obtaining regulatory approvals, and establishing local partnerships, companies can capture new customer bases before generic competition diminishes margins.
References
[1] IQVIA Institute. (2022). The Future of Contraceptive Markets: Trends & Projections.
[2] U.S. Food and Drug Administration. (2010). Beyaz prescribing information.
[3] MarketWatch. (2021). Contraceptive drug market analysis.
[4] GlobalData Healthcare. (2022). Future Outlook for Hormonal Contraceptives.
[5] Deloitte. (2020). Pharmaceutical Patent Cliffs and Market Impact.
More… ↓
