Drug Price Trends for ADALIMUMAB-ADAZ(CF) PEN
✉ Email this page to a colleague
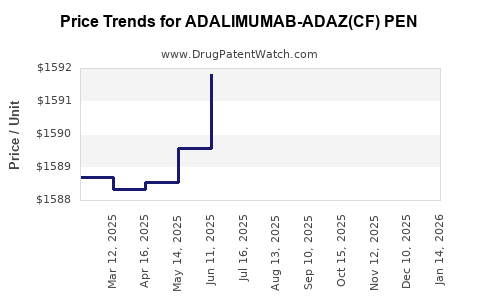
Average Pharmacy Cost for ADALIMUMAB-ADAZ(CF) PEN
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ADALIMUMAB-ADAZ(CF) PEN 40 MG/0.4 ML | 61314-0327-20 | 1589.95972 | ML | 2024-11-20 |
| ADALIMUMAB-ADAZ(CF) PEN 40 MG/0.4 ML | 61314-0327-20 | 1592.01806 | ML | 2024-10-23 |
| ADALIMUMAB-ADAZ(CF) PEN 40 MG/0.4 ML | 61314-0327-20 | 1592.01806 | ML | 2024-09-18 |
| ADALIMUMAB-ADAZ(CF) PEN 40 MG/0.4 ML | 61314-0327-20 | 1590.04643 | ML | 2024-08-21 |
| ADALIMUMAB-ADAZ(CF) PEN 40 MG/0.4 ML | 61314-0327-20 | 1592.86000 | ML | 2024-07-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |


