Share This Page
Drug Price Trends for PREDNISOLONE SOD
✉ Email this page to a colleague
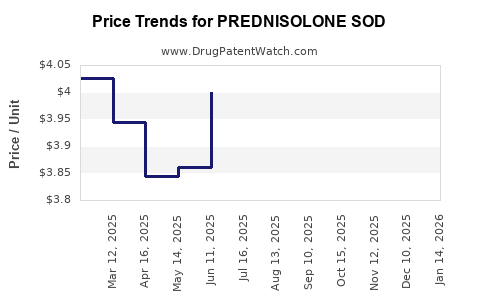
Average Pharmacy Cost for PREDNISOLONE SOD
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| PREDNISOLONE SOD 1% EYE DROP | 24208-0715-10 | 4.43607 | ML | 2025-12-17 |
| PREDNISOLONE SOD 1% EYE DROP | 24208-0715-10 | 4.37507 | ML | 2025-11-19 |
| PREDNISOLONE SOD 1% EYE DROP | 24208-0715-10 | 4.35826 | ML | 2025-10-22 |
| PREDNISOLONE SOD 1% EYE DROP | 24208-0715-10 | 4.30656 | ML | 2025-09-17 |
| PREDNISOLONE SOD 1% EYE DROP | 24208-0715-10 | 4.28592 | ML | 2025-08-20 |
| PREDNISOLONE SOD PH 25 MG/5 ML | 42799-0816-01 | 0.78812 | ML | 2025-07-23 |
| PREDNISOLONE SOD PH 25 MG/5 ML | 44523-0182-08 | 0.78812 | ML | 2025-07-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Prednisolone Sodium Phosphate (Prednisolone Sod)
Introduction
Prednisolone sodium phosphate (Prednisolone Sod) is a synthetic corticosteroid used primarily for its anti-inflammatory and immunosuppressive effects. It is indicated for a broad spectrum of conditions including allergic reactions, respiratory diseases, and autoimmune disorders. Given its widespread clinical use, analyzing its market dynamics and projecting future pricing trends is vital for pharmaceutical companies, healthcare providers, and investors. This report offers a comprehensive market analysis, evaluates current pricing strategies, and forecasts future price movements for Prednisolone Sod.
Market Landscape
Global Market Overview
The corticosteroids market, encompassing drugs like Prednisolone Sod, is projected to experience sustained growth driven by rising prevalence of autoimmune and inflammatory diseases. As of 2022, the global corticosteroids market was valued at approximately USD 3.5 billion, with Prednisolone constituting a significant segment owing to its patent expirations and the resulting proliferation of generic versions [1].
Prednisolone Sod's prominence stems from several factors:
- Clinical Efficacy and Versatility: Its efficacy across diverse indications makes it a staple in treatment protocols.
- Availability of Generics: Patent expiry has led to increased generic manufacturing, boosting accessibility and usage.
- Global Demand: High demand in North America, Europe, and Asia-Pacific, driven by aging populations and expanding healthcare infrastructure.
Regional Market Dynamics
- North America: The largest market, accounting for roughly 45% of sales, due to high prevalence of autoimmune conditions, established healthcare infrastructure, and widespread usage of corticosteroids.
- Europe: Substantial market share with a focus on autoimmune and inflammatory conditions, coupled with a mature generic drug market.
- Asia-Pacific: Rapidly growing demand facilitated by expanding healthcare access, aging populations, and increasing autoimmune disorder incidence.
Market Drivers and Challenges
Drivers:
- Rising incidence of autoimmune, allergic, and respiratory diseases.
- Growing geriatric population susceptible to chronic inflammatory conditions.
- Cost-effective generic alternatives increasing accessibility.
Challenges:
- Stringent regulatory requirements for manufacturing and approvals.
- Potential side effects prompting cautious prescribing patterns.
- Competition from newer corticosteroids or biologics for specific indications.
Competitive Landscape
The market is highly commoditized post-patent expiry, with numerous generic manufacturers globally. Key players include:
- Teva Pharmaceuticals
- Mylan (now part of Viatris)
- Pfizer
- Sandoz (Novartis)
- Sun Pharma
Brand variants are limited; most market share resides with generic versions. Pricing competitiveness, manufacturing efficiency, and distribution channels influence market positioning.
Pricing Analysis
Current Pricing Trends
The price of Prednisolone Sod varies significantly across regions, drug formulations, and suppliers. In the United States, the average retail price for a standard 5 mg tablet ranges between USD 0.02 and USD 0.05 per tab, reflecting generic competition and procurement volume discounts [2].
In European markets, the per-unit cost is comparable, with regional pricing regulations impacting retail and hospital procurement. In Asia-Pacific, prices are often lower due to local manufacturing and competitive pricing strategies, with tablets sometimes costing less than USD 0.01 per unit.
Factors Influencing Pricing
- Patent Status: As a generic drug, Prednisolone Sod's prices are driven by manufacturing costs, market competition, and regulatory costs.
- Manufacturing Costs: Bulk production efficiencies and raw material costs influence the minimum sustainable price.
- Regulatory Environment: Regulatory compliance costs can affect pricing, especially in strict jurisdictions like the US and EU.
- Reimbursement Policies: Insurance reimbursement rates and government policies also impact final consumer prices.
- Market Penetration Strategies: Distributors may adopt aggressive discounting to gain market share, regulating prices downward.
Future Price Projections
Short-term Outlook (Next 1-2 years)
- Pricing Stability: As the market matures with an oversupply of generic options, prices for Prednisolone Sod are expected to stabilize or decline modestly, especially in developed countries.
- Cost Pressures: Raw material inflation and manufacturing costs could marginally influence prices, but competitive pressures will limit significant increases.
Medium to Long-term Outlook (3-5 years)
- Price Compression: Continued market saturation and evolving procurement strategies might drive prices downward by approximately 5-10% annually.
- Generic Market Consolidation: Increased market consolidation or manufacturing shifts could lead to slight price fluctuations.
- Emerging Markets: In emerging economies, price declines may be less pronounced or stagnate due to local regulations and supply chain constraints.
Influence of Regulatory and Policy Changes
- Stricter biosimilar and generic regulations could impact market dynamics, possibly encouraging price reductions or stalling price increases.
- The potential approval of new, more effective corticosteroid formulations or biologics for specific indications could exert downward pricing pressure on Prednisolone Sod.
Economic and Market Considerations
- The increasing adoption of value-based healthcare models emphasizes cost-effectiveness, further incentivizing low-cost generics.
- Supply chain disruptions, such as those observed during the COVID-19 pandemic, could temporarily influence prices due to raw material shortages.
- The rising emphasis on sustainable manufacturing practices might lead to increased costs, slightly affecting prices.
Regulatory Environment Impact
Regulations influence market entry, pricing policies, and reimbursement. Countries with centralized procurement systems (e.g., the EU) tend to have more controlled pricing, while liberal markets like the US display more variability. Policy shifts toward drug price negotiation, especially in the US, could lead to significant reductions in Prednisolone Sod's price [3].
Conclusion
The Prednisolone Sod market is characterized by intense competition, broad global demand, and a predominantly generic landscape. Price stability in developed markets is expected to continue in the short term, with gradual declines over the next five years driven by market saturation, regulatory factors, and healthcare cost-containment policies. Emerging markets may see less price compression due to local manufacturing and regulatory dynamics. Stakeholders should monitor regulatory developments and supply chain trends to adapt pricing strategies accordingly.
Key Takeaways
- Market Drivers: Increasing prevalence of autoimmune and inflammatory diseases sustains global demand for Prednisolone Sod.
- Pricing Trends: Prices are currently stable with ongoing downward pressure driven by generic competition and market saturation.
- Future Outlook: Expect gradual price declines (5-10% annually over 3-5 years) influenced by regulatory policies, healthcare cost pressures, and generic market dynamics.
- Strategic Focus: Manufacturers should optimize cost efficiencies, monitor regulatory changes, and explore emerging markets for growth opportunities.
- Regulatory Impact: Policy changes, particularly around drug negotiations and reimbursement, could accelerate price adjustments.
FAQs
1. How does patent expiry influence the price of Prednisolone Sod?
Patent expiry introduces multiple generic competitors, significantly reducing prices due to increased market competition and manufacturing efficiencies.
2. What factors could lead to a sharp rise in Prednisolone Sod prices in the future?
Regulatory changes restricting generic formulations, supply chain disruptions, or increased raw material costs could cause spikes in pricing.
3. How do regional healthcare policies impact Prednisolone Sod pricing?
Price controls, reimbursement policies, and procurement practices vary regionally, affecting retail and hospital purchase prices.
4. Are there any new formulations of corticosteroids likely to replace Prednisolone Sod?
While new biologic agents and targeted therapies are emerging, Prednisolone Sod remains a cost-effective and widely used corticosteroid. Replacement would depend on clinical efficacy, safety, and approval pathways.
5. What strategies can manufacturers employ to maintain profitability amidst declining prices?
Cost reduction through manufacturing efficiencies, diversification of product portfolios, focus on emerging markets, and value-added services can help sustain profitability.
References
- MarketWatch. “Corticosteroids Market Size, Share & Trends Analysis Report.” 2022.
- GoodRx. “Prednisolone Prices and Cost Comparison.” 2023.
- FDA Press Releases. “Prezidential Negotiation Policies and Drug Pricing Trends.” 2022.
More… ↓
