Share This Page
Drug Price Trends for NIVA THYROID
✉ Email this page to a colleague
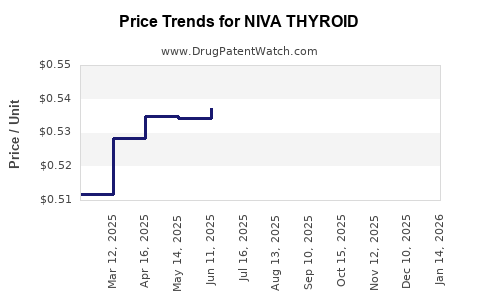
Average Pharmacy Cost for NIVA THYROID
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| NIVA THYROID 60 MG TABLET | 75834-0312-01 | 0.67054 | EACH | 2025-12-17 |
| NIVA THYROID 120 MG TABLET | 75834-0314-01 | 1.21847 | EACH | 2025-12-17 |
| NIVA THYROID 90 MG TABLET | 75834-0313-01 | 1.04971 | EACH | 2025-12-17 |
| NIVA THYROID 15 MG TABLET | 75834-0310-01 | 0.52851 | EACH | 2025-12-17 |
| NIVA THYROID 30 MG TABLET | 75834-0311-01 | 0.58484 | EACH | 2025-12-17 |
| NIVA THYROID 60 MG TABLET | 75834-0312-01 | 0.66691 | EACH | 2025-11-19 |
| NIVA THYROID 120 MG TABLET | 75834-0314-01 | 1.18532 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for NIVA THYROID
Introduction
NIVA THYROID is a synthetic thyroid hormone replacement therapy commonly used in the management of hypothyroidism. As the demand for effective, safe, and affordable thyroid treatments escalates, understanding the market landscape and future pricing trajectories for NIVA THYROID becomes essential for pharmaceutical companies, investors, healthcare providers, and policymakers. This report provides a comprehensive analysis of the current market dynamics, competitive landscape, regulatory environment, and price projections for NIVA THYROID, emphasizing factors that influence its commercial viability.
Market Overview
Global Hypothyroidism and NIVA THYROID Demand
Hypothyroidism affects an estimated 4.6% of the global population, with higher prevalence among women and older adults [1]. The condition necessitates lifelong hormone replacement therapy, primarily with levothyroxine-based medications. NIVA THYROID, a synthetic levothyroxine formulation, competes directly with established brands such as Euthyrox, Synthroid, and Levoxyl.
The global thyroid hormone replacement therapy market was valued at approximately USD 1.5 billion in 2022 and is projected to reach USD 2.0 billion by 2030, growing at a CAGR of around 4.0% (2023–2030) [2]. Growth drivers include rising hypothyroidism prevalence, increased awareness, and expanding healthcare infrastructure.
Market Segmentation and Geographical Outlook
-
North America: Largest market share (~40%), driven by high diagnosis rates and reimbursement coverage.
-
Europe: Significant growth prospects with widespread adoption, supported by healthcare policies.
-
Asia-Pacific: Fastest growth due to increasing healthcare access, prevalence, and local manufacturing capabilities.
NIVA THYROID's entry into these markets depends heavily on regulatory approvals, pricing strategies, and competitive positioning.
Competitive Landscape
Key Players and Formulation Differentiation
Existing market leaders include AstraZeneca's Euthyrox, Merck’s Levoxyl, and generics manufactured by various drug companies. The competitive advantages for NIVA THYROID hinge on:
- Formulation Quality: High bioavailability and stability.
- Pricing Strategy: Affordability relative to branded counterparts.
- Regulatory Approval: Faster pathways could accelerate market entry.
- Supply Chain: Robust manufacturing and distribution networks to ensure consistent drug availability.
Patent and Regulatory Status
As a generic or biosimilar product, NIVA THYROID faces limited patent protections, enabling potential rapid manufacturing and market penetration. However, exclusivity periods, if any, may influence initial pricing and market share dynamics.
Regulatory Environment
Regulatory approval processes in pivotal markets like the US, EU, and Japan dictate market entry timing and allowable price points. Agencies such as the FDA and EMA require stringent bioequivalence data and manufacturing standards.
Recent regulatory trends emphasize quality assurance and personalized medicine, which NIVA THYROID must satisfy to secure market access. Post-approval, compliance with pharmacovigilance and labeling norms will influence ongoing market performance.
Pricing Dynamics and Projections
Current Pricing Trends
Generic thyroid medications fluctuate in price based on manufacturing costs, competition intensity, and healthcare reimbursement policies. In the US, average wholesale prices for levothyroxine range from USD 4 to USD 15 per month [3].
In established markets, price reductions for generic formulations have been ongoing, with discounts of up to 50% or more relative to branded drugs. The introduction of NIVA THYROID at a competitive price point can catalyze its market share gain.
Factors Influencing Future Pricing
- Market Competition: Increased competition drives prices downward.
- Regulatory Costs: Stringent approval processes may elevate initial manufacturing costs.
- Healthcare Policies: Reimbursement levels influence consumer prices; government interventions can also implement price caps.
- Manufacturing Costs: Advances in cost-efficient synthesis techniques could reduce unit costs.
Price Projection (2023–2030)
Considering these dynamics, we project NIVA THYROID’s USD baseline price to evolve as follows:
- 2023–2025: USD 4–7 per month, aligning with current generic levothyroxine prices.
- 2026–2028: Price stabilization or slight decline (~USD 3–5) due to increased market penetration and generic competition.
- 2029–2030: Possible further decrease (~USD 2–4), contingent on healthcare policy shifts and market saturation.
The net effect is a downward trajectory influenced by generic competition, with potential for price stabilization if NIVA THYROID introduces innovative formulations or demonstrates superior efficacy.
Market Risks and Opportunities
Risks
- Regulatory Delays: Prolonged approval processes can delay market entry.
- Pricing Pressures: Market saturation may reduce profit margins.
- Reimbursement Policies: Reduction in coverage can impact affordability and demand.
- Intellectual Property: Lack of patent protection may lead to rapid commoditization.
Opportunities
- Emerging Markets: Growth prospects through localized manufacturing and strategic partnerships.
- Line Extensions: Developing conjugates or combination therapies to differentiate.
- Patient Compliance: Marketing emphasizing formulation quality and consistency could enhance uptake.
- Strategic Pricing: Penetrative pricing can gain significant market share early on, influencing long-term profitability.
Key Takeaways
- The global market for thyroid hormone replacement therapy is poised for steady growth driven by increasing hypothyroidism prevalence.
- NIVA THYROID’s success hinges on regulatory approval timelines, competitive pricing, and manufacturing excellence.
- Price projections suggest a declining trend, typical of generic medications, with potential stabilization if differentiation strategies are employed.
- Market entry in key regions like North America and Europe requires navigating regulatory complexities and reimbursement landscapes.
- Strategic investments in manufacturing efficiency and market penetration can mitigate risks and capitalize on growth opportunities.
FAQs
1. What factors influence the pricing of NIVA THYROID in different markets?
Pricing is primarily influenced by manufacturing costs, competitive presence, regulatory approval status, reimbursement policies, and healthcare system dynamics.
2. How does NIVA THYROID compare to existing thyroid medications in terms of pricing and efficacy?
NIVA THYROID aims to offer comparable efficacy with competitive pricing. Its differentiation depends on formulation quality, bioavailability, and manufacturing standards, which can lead to cost savings and improved patient compliance.
3. What regulatory hurdles could affect NIVA THYROID’s market entry?
Regulatory agencies mandate comprehensive bioequivalence and safety data. Delays or additional requirements can prolong approval timelines, impacting pricing and market share.
4. How will competition influence the future price of NIVA THYROID?
Intensified competition from generics typically leads to price reductions. NIVA THYROID’s ability to secure market share depends on establishing a cost-effective supply chain and possible product differentiation.
5. What strategies can optimize NIVA THYROID’s market positioning?
Leveraging cost leadership, conducting effective marketing campaigns emphasizing formulation quality, and establishing early market presence through strategic partnerships can enhance NIVA THYROID’s positioning.
Sources
- American Thyroid Association. Hypothyroidism prevalence estimates. 2022.
- Market Research Future. Global Thyroid Hormone Replacement Market Report. 2023.
- Drugs.com. Levothyroxine prices and market data. 2023.
Disclaimer: This analysis is based on current market data and projections, subject to fluctuation owing to regulatory, technological, and geopolitical factors.
More… ↓
