DrugPatentWatch's Custom Market Surveillance provides you with a robust platform to monitor your business opportunities from every angle.
You're monitoring your business, but you're too busy to sift through data.
You need a snapshot of all your business information and data.
Our intelligent tools and reports can help you and make your life easier.
You can use adaptive reports and visualization tools to simplify your monitoring processes. It will help you focus on the right areas and determine where to investigate first.
How Can DrugPatentWatch Custom Market Surveillance Help You?
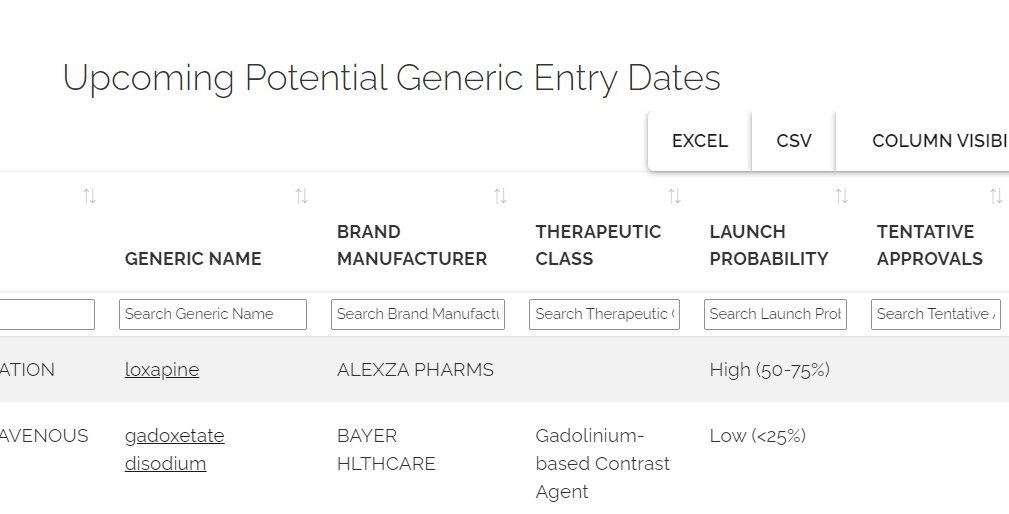
Anticipate generic entry opportunities
- Track the likelihood of generic launch
- Identify potential first generic entrants
- Track litigation to anticipate early generic entry
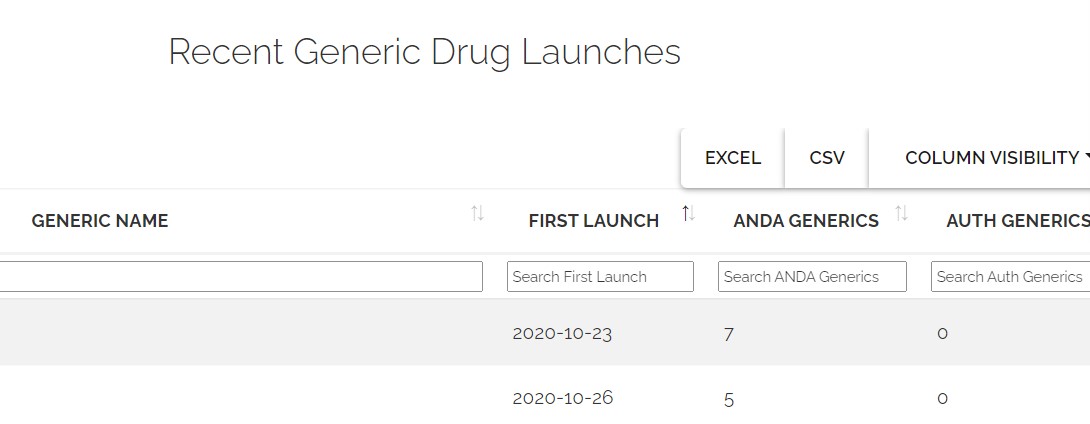
Track Generic Entry Momentum
- Monitor authorized generic activity
- Anticipate price changes as more generics launch
- Assess competitive landscape
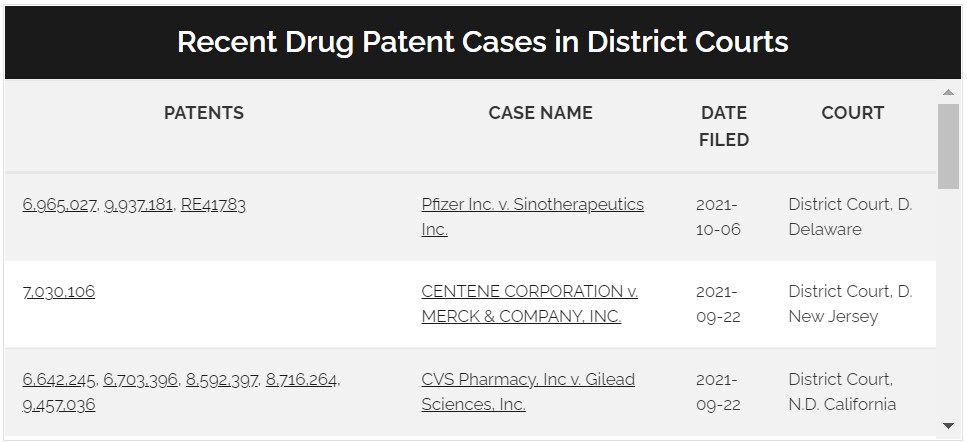
Watch Drug Patent Litigation
- Track litigation to anticipate early generic entry
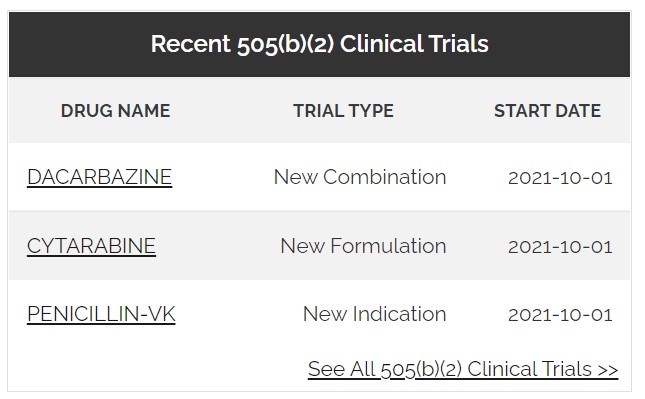
Monitor biosimilar and 505(b)(2) activity
- Anticipate 505(b)(2) and biosimilar approvals
- Track OTC-switches, new formulations, biosimilars, and other drug improvements
- Strengthen new formulation patents by studying prior claims and litigation
Email Alerts
- Get notice of critical events, directly in your inbox


