PERTZYE Drug Profile
✉ Email this page to a colleague
Summary for Tradename: PERTZYE
| High Confidence Patents: | 7 |
| Applicants: | 1 |
| BLAs: | 1 |
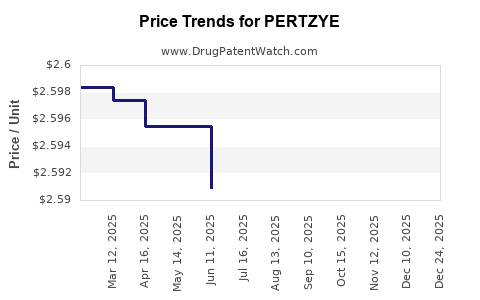
| Drug Prices: | Drug price information for PERTZYE |
| Recent Clinical Trials: | See clinical trials for PERTZYE |
Recent Clinical Trials for PERTZYE
Identify potential brand extensions & biosimilar entrants
| Sponsor | Phase |
|---|---|
| Memorial Sloan Kettering Cancer Center | Phase 1/Phase 2 |
| Digestive Care, Inc. | Phase 1/Phase 2 |
| Massimo Raimondo, M.D. | Phase 1/Phase 2 |
Note on Biologic Patents
Matching patents to biologic drugs is far more complicated than for small-molecule drugs.
DrugPatentWatch employs three methods to identify biologic patents:
- Brand-side disclosures in response to biosimilar applications
- DrugPatentWatch analysis and company disclosures
- Patents from broad patent text search
These patents were identified from disclosures by the brand-side company, in response to a potential biosimilar seeking to launch. They have a high certainty of blocking biosimilar entry. The expiration dates listed are not estimates — they're expiration dates as indicated by the brand-side company.
These patents were identified from searching various sources, including drug labels and other general disclosures from the brand-side company. This list may exclude some of the patents which block biosimilar launch, and some of these patents listed may not actually block biosimilar launch. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
For completeness, these patents were identified by searching the patent literature for mentions of the branded or ingredient name of the drug. Some of these patents protect the original drug, whereas others may protect follow-on inventions or even inventions casually mentioning the drug. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
1) High Certainty: US Patents for PERTZYE Derived from Brand-Side Litigation
No patents found based on brand-side litigation
2) High Certainty: US Patents for PERTZYE Derived from DrugPatentWatch Analysis and Company Disclosures
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2012-06-22 | DrugPatentWatch analysis and company disclosures |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2012-06-22 | DrugPatentWatch analysis and company disclosures |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2013-08-11 | DrugPatentWatch analysis and company disclosures |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2013-09-29 | DrugPatentWatch analysis and company disclosures |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2015-05-04 | DrugPatentWatch analysis and company disclosures |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2016-05-29 | DrugPatentWatch analysis and company disclosures |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
3) Low Certainty: US Patents for PERTZYE Derived from Patent Text Search
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2035-05-08 | Patent claims search |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2036-11-30 | Patent claims search |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2029-03-09 | Patent claims search |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2035-06-19 | Patent claims search |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2033-10-02 | Patent claims search |
| Digestive Care, Inc. | PERTZYE | pancrelipase | Capsule, Delayed Release | 022175 | ⤷ Get Started Free | 2037-06-14 | Patent claims search |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
International Patents for PERTZYE
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Taiwan | I609693 | ⤷ Get Started Free |
| European Patent Office | 2079445 | ⤷ Get Started Free |
| Chile | 2008000517 | ⤷ Get Started Free |
| Hungary | E026168 | ⤷ Get Started Free |
| Malaysia | 163469 | ⤷ Get Started Free |
| Israel | 200407 | ⤷ Get Started Free |
| >Country | >Patent Number | >Estimated Expiration |
Market Dynamics and Financial Trajectory for PERTZYE
More… ↓