Harmony Company Profile
✉ Email this page to a colleague
What is the competitive landscape for HARMONY, and when can generic versions of HARMONY drugs launch?
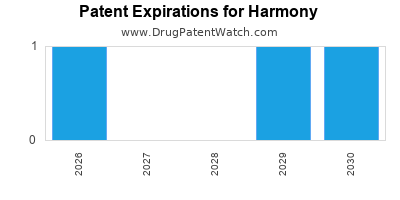
HARMONY has one approved drug.
There are three US patents protecting HARMONY drugs.
There are fifty-three patent family members on HARMONY drugs in thirty-one countries and ten supplementary protection certificates in nine countries.
Drugs and US Patents for Harmony
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | RX | Yes | Yes | 8,354,430 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Harmony
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | 7,169,928 | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | 7,169,928 | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-001 | Aug 14, 2019 | 7,910,605 | ⤷ Try a Trial |
| Harmony | WAKIX | pitolisant hydrochloride | TABLET;ORAL | 211150-002 | Aug 14, 2019 | 7,910,605 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Harmony Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Montenegro | 01713 | ⤷ Try a Trial |
| Slovenia | 1846384 | ⤷ Try a Trial |
| South Africa | 200708086 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006084833 | ⤷ Try a Trial |
| European Patent Office | 1863487 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Harmony Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1428820 | SPC/GB16/052 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, SUCH AS HYDROCHLORIDE; REGISTERED: UK EU/1/15/1068 20160404 |
| 1428820 | 2016C/048 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES TELS QUE LE CHLORHYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/15/1068 20160404 |
| 1428820 | 300832 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN, ZOALS HET HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/15/1068/002 20160404 |
| 1428820 | CA 2016 00042 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT OG FARMACEUTISK ACCEPTABLE SALTE HERAF, SASOM PITOLISANT HYDROCHLORID; REG. NO/DATE: EU/1/15/1068 20160404 |
| 1428820 | 16C0038 | France | ⤷ Try a Trial | PRODUCT NAME: PITOLISANT ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES TELS QUE LE CHLORHYDRATE; REGISTRATION NO/DATE: EU/1/15/1068 20160331 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.