[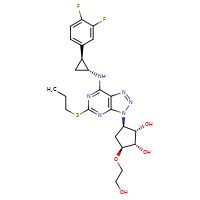](//www.DrugPatentWatch.com/p/preview/generic-api/ticagrelor?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog)
Ticagrelor is the generic ingredient in two branded drugs marketed by Astrazeneca Pharms, Amneal, Hisun Pharm Hangzhou, Sigmapharm Labs Llc, Sunshine, and Watson Labs Inc and, and is included in six NDAs. There are four patents protecting this compound.
Drug patent litigation for TICAGRELOR.
This ingredient has two hundred and one patent family members in forty-eight countries.
Three suppliers are listed for this compound. There are five tentative approvals for this compound.
For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit www.DrugPatentWatch.com
