Glycopyrrolate - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for glycopyrrolate and what is the scope of patent protection?
Glycopyrrolate
is the generic ingredient in ten branded drugs marketed by Abraxis Pharm, Accord Hlthcare, Alembic, Am Regent, Amneal, Apotex, Aspen, Caplin, Eugia Pharma, Fresenius Kabi Usa, Gland Pharma Ltd, Hikma Farmaceutica, Hospira, Lupin Ltd, Mankind Pharma, Meitheal, Nivagen Pharms Inc, Piramal Critical, Prinston Inc, Sagent, Sandoz, Somerset Theraps Llc, Teva Parenteral, Watson Labs, Xiromed, Zydus Pharms, Hikma, Robins Ah, Novartis, Sumitomo Pharma Am, Exela Pharma, Merz Pharms, Annora Pharma, Par Pharm Inc, Suven Pharms, Edenbridge Pharms, Aurobindo Pharma, Chartwell Rx, Dr Reddys Labs Ltd, Heritage Pharms Inc, Hikma Intl Pharms, Indoco, Leading, Lgm Pharma, Natco, Oxford Pharms, Par Pharm, Rising, Sun Pharm Inds Ltd, Casper Pharma Llc, and Azurity, and is included in sixty-one NDAs. There are sixteen patents protecting this compound and four Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Glycopyrrolate has one hundred and seventy-five patent family members in thirty-two countries.
There are seventeen drug master file entries for glycopyrrolate. Fifty-five suppliers are listed for this compound.
Summary for glycopyrrolate
| International Patents: | 175 |
| US Patents: | 16 |
| Tradenames: | 10 |
| Applicants: | 51 |
| NDAs: | 61 |
| Drug Master File Entries: | 17 |
| Finished Product Suppliers / Packagers: | 55 |
| Raw Ingredient (Bulk) Api Vendors: | 113 |
| Clinical Trials: | 153 |
| Patent Applications: | 6,853 |
| Formulation / Manufacturing: | see details |
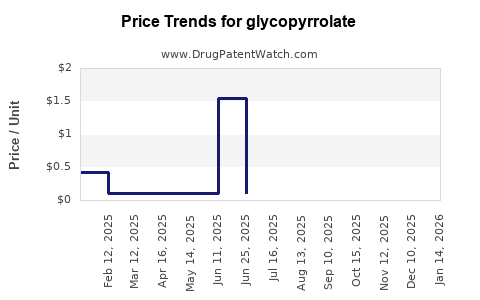
| Drug Prices: | Drug price trends for glycopyrrolate |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for glycopyrrolate |
| What excipients (inactive ingredients) are in glycopyrrolate? | glycopyrrolate excipients list |
| DailyMed Link: | glycopyrrolate at DailyMed |
Recent Clinical Trials for glycopyrrolate
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Trauma Center | N/A |
| Merck Sharp & Dohme LLC | Phase 4 |
| Ciusss de L'Est de l'Île de Montréal | N/A |
Pharmacology for glycopyrrolate
| Drug Class | Anticholinergic Cholinergic Muscarinic Antagonist |
| Mechanism of Action | Cholinergic Antagonists Cholinergic Muscarinic Antagonists |
Medical Subject Heading (MeSH) Categories for glycopyrrolate
Paragraph IV (Patent) Challenges for GLYCOPYRROLATE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| CUVPOSA | Oral Solution | glycopyrrolate | 1 mg/5 mL | 022571 | 1 | 2012-06-20 |
| ROBINUL FORTE | Tablets | glycopyrrolate | 2 mg | 012827 | 1 | 2010-10-12 |
| ROBINUL FORTE | Tablets | glycopyrrolate | 1 mg | 012827 | 1 | 2009-08-14 |
US Patents and Regulatory Information for glycopyrrolate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exela Pharma | GLYRX-PF | glycopyrrolate | SOLUTION;INTRAMUSCULAR, INTRAVENOUS | 210997-001 | Jul 11, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sagent | GLYCOPYRROLATE | glycopyrrolate | INJECTABLE;INJECTION | 210083-001 | Feb 21, 2020 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Meitheal | GLYCOPYRROLATE | glycopyrrolate | INJECTABLE;INJECTION | 212802-001 | Jul 6, 2021 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Casper Pharma Llc | ROBINUL FORTE | glycopyrrolate | TABLET;ORAL | 012827-002 | Approved Prior to Jan 1, 1982 | AA | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Gland Pharma Ltd | GLYCOPYRROLATE | glycopyrrolate | INJECTABLE;INJECTION | 212612-001 | Sep 30, 2019 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Alembic | GLYCOPYRROLATE | glycopyrrolate | TABLET;ORAL | 203657-001 | Nov 30, 2018 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for glycopyrrolate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | SEEBRI | glycopyrrolate | POWDER;INHALATION | 207923-001 | Oct 29, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | SEEBRI | glycopyrrolate | POWDER;INHALATION | 207923-001 | Oct 29, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | SEEBRI | glycopyrrolate | POWDER;INHALATION | 207923-001 | Oct 29, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Casper Pharma Llc | ROBINUL | glycopyrrolate | TABLET;ORAL | 012827-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | SEEBRI | glycopyrrolate | POWDER;INHALATION | 207923-001 | Oct 29, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sumitomo Pharma Am | LONHALA MAGNAIR KIT | glycopyrrolate | SOLUTION;INHALATION | 208437-001 | Dec 5, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for glycopyrrolate
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | I353857 | ⤷ Try a Trial | |
| European Patent Office | 2883564 | Dispositif d'administration d'aérosol et procédé de fonctionnement dudit dispositif (Aerosol delivery device and method of operating the aerosol delivery device) | ⤷ Try a Trial |
| Japan | 2018525094 | エアロゾル発生器のための振動可能ヘッドを製造する方法およびエアロゾル発生器のための振動可能ヘッド | ⤷ Try a Trial |
| Israel | 178878 | מתקן משאף (Inhaler device) | ⤷ Try a Trial |
| China | 1802157 | Pharmaceutical compositions comprising apomorphine for pulmonary inhalation | ⤷ Try a Trial |
| Japan | 2006522785 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for glycopyrrolate
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435024 | 21C1020 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI), GLYCOPYRROLATE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET BUDESONIDE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 2435024 | 2021C/518 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE FORMOTEROL (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), GLYCOPYRROLATE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET BUDESONIDE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1498 20201210 |
| 2435024 | 2190014-7 | Sweden | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF FORMOTEROL INCLUDING ANY PHARMACEUUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, GLYCOPYRROLATE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS, OR SOLVATES THEREOF, AND BUDESONIDE INCLUDING ANY PHARMACEUTICALLY ACCEPTABLE SALT, ESTERS ORSOLVATES THEREOF; REG. NO/DATE: EU/1/20/1498 20201210 |
| 2435024 | SPC/GB21/029 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF FORMOTEROL, INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS AND SOLVATES THEREOF, GLYCOPYRROLATE, INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ESTERS AND SOLVATES THEREOF, AND BUDESONIDE INCLUDING PHARMACEUTICALLY ACCEPTABLE SALTS, ES; REGISTERED: UK EU/1/20/1498 (NI) 20201210; UK PLGB 17901/0352-001 20201210 |
| 2435025 | LUC00124 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS SES SELS, ESTERS, ENANTIOMERES OU AUTRES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, ENANTIOMERES OU AUTRES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/18/1339 20181220 |
| 2435025 | 19C1040 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET DE FORMOTEROL (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); NAT. REGISTRATION NO/DATE: EU/1/18/1339 20181220; FIRST REGISTRATION: - EU/1/18/1339 20181220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.