Amneal Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AMNEAL, and when can generic versions of AMNEAL drugs launch?
AMNEAL has three hundred and eighty-four approved drugs.
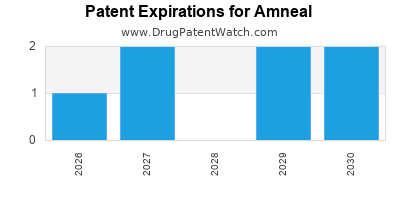
There are five US patents protecting AMNEAL drugs. There are seven tentative approvals on AMNEAL drugs.
There are fifteen patent family members on AMNEAL drugs in twelve countries and seven hundred and ninety-nine supplementary protection certificates in eighteen countries.
Summary for Amneal
| International Patents: | 15 |
| US Patents: | 5 |
| Tradenames: | 291 |
| Ingredients: | 283 |
| NDAs: | 384 |
| Patent Litigation for Amneal: | See patent lawsuits for Amneal |
| PTAB Cases with Amneal as petitioner: | See PTAB cases with Amneal as petitioner |
Drugs and US Patents for Amneal
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amneal Pharms Co | DOXEPIN HYDROCHLORIDE | doxepin hydrochloride | CAPSULE;ORAL | 207482-002 | Jun 28, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharm | BETHANECHOL CHLORIDE | bethanechol chloride | TABLET;ORAL | 040855-003 | Nov 21, 2007 | AA | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms | SUCRALFATE | sucralfate | TABLET;ORAL | 215576-001 | Apr 15, 2022 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms Co | ETHACRYNIC ACID | ethacrynic acid | TABLET;ORAL | 208805-001 | May 8, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms | PROMETHAZINE HYDROCHLORIDE | promethazine hydrochloride | SYRUP;ORAL | 040882-001 | Dec 30, 2009 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Amneal | THEOPHYLLINE | theophylline | TABLET, EXTENDED RELEASE;ORAL | 216276-001 | Mar 20, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal | METHYLPREDNISOLONE | methylprednisolone | TABLET;ORAL | 207481-001 | Sep 21, 2021 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Amneal
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 5,466,699*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 6,750,237*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 6,750,237*PED | ⤷ Try a Trial |
| Amneal | ACTIVELLA | estradiol; norethindrone acetate | TABLET;ORAL | 020907-001 | Nov 18, 1998 | RE36247 | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 7,220,767*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 7,220,767*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for AMNEAL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Nasal Spray | 2.5 mg/spray | ➤ Subscribe | 2016-06-09 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Nasal Spray | 5 mg/spray | ➤ Subscribe | 2013-11-14 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
International Patents for Amneal Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Russian Federation | 2015100116 | ⤷ Try a Trial |
| Mexico | 2022001139 | ⤷ Try a Trial |
| European Patent Office | 2881109 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2021021277 | ⤷ Try a Trial |
| Canada | 2879383 | ⤷ Try a Trial |
| Spain | 2795421 | ⤷ Try a Trial |
| Australia | 2020323846 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Amneal Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3043773 | 21C1057 | France | ⤷ Try a Trial | PRODUCT NAME: MOMETASONE OU L'UN DE SES SELS AVEC OLOPATADINE OU L'UN DE SES SELS; NAT. REGISTRATION NO/DATE: NL52121 20211026; FIRST REGISTRATION: AT - 140638 20210426 |
| 1261586 | 2012C/016 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE PRODUITS DE SAXAGLIPTINE ET DE METFORMINE AINSI QUE TOUT SELS PHARMACEUTIQUEMENT ACCEPTABLES, DONT LES SELS DE CHLORHYDRATE DE SAXAGLIPTINE ET DE METFORMINE; AUTHORISATION NUMBER AND DATE: EU/1/11/731/001 20111128 |
| 0463756 | 300048 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ZOLMITRIPTANUM, DESGEWENST IN DE VORM VAN EEN FYSIOLOGISCH AANVAARDBAAR ZOUT OF VAN EEN SOLVAAT; NATL. REGISTRATION: RVG 21079 RVG 21080 19970925; FIRST REGISTRATION: PL 12619/0116 19970307 |
| 2137537 | 122014000069 | Germany | ⤷ Try a Trial | PRODUCT NAME: DIMETHYLFUMARAT; REGISTRATION NO/DATE: EU/1/13/837/001-002 20140130 |
| 2962690 | CA 2019 00033 | Denmark | ⤷ Try a Trial | PRODUCT NAME: APREMILAST ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/14/981 20150116 |
| 0591280 | C300103 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FROVATRIPTAN EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; NAT. REGISTRATION NO/DATE: RVG 27211RVG 27212 2002190419; FIRST REGISTRATION: NL 24548 20001212 |
| 0716606 | SPC/GB02/011 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER; REGISTERED: UK EU/1/99/120/001-004 20000202 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.