Azurity Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AZURITY, and when can generic versions of AZURITY drugs launch?
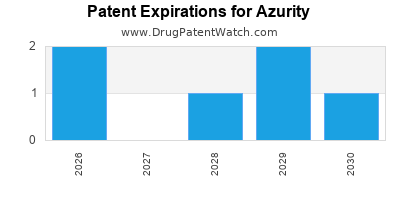
AZURITY has twenty-three approved drugs.
There are seventy-eight US patents protecting AZURITY drugs.
There are three hundred and thirty-five patent family members on AZURITY drugs in forty-eight countries and forty-one supplementary protection certificates in fifteen countries.
Summary for Azurity
| International Patents: | 335 |
| US Patents: | 78 |
| Tradenames: | 23 |
| Ingredients: | 22 |
| NDAs: | 23 |
| Patent Litigation for Azurity: | See patent lawsuits for Azurity |
Drugs and US Patents for Azurity
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azurity | EPANED | enalapril maleate | SOLUTION;ORAL | 208686-001 | Sep 20, 2016 | AB | RX | Yes | Yes | 9,808,442 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Azurity | EDARBYCLOR | azilsartan kamedoxomil; chlorthalidone | TABLET;ORAL | 202331-001 | Dec 20, 2011 | RX | Yes | No | 7,572,920 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Azurity | EVEKEO ODT | amphetamine sulfate | TABLET, ORALLY DISINTEGRATING;ORAL | 209905-001 | Jan 30, 2019 | RX | Yes | No | 11,160,772 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Azurity | NYMALIZE | nimodipine | SOLUTION;ORAL | 203340-002 | Apr 8, 2020 | RX | Yes | Yes | 8,517,997 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Azurity
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | 5,583,141 | ⤷ Try a Trial |
| Azurity | EVEKEO ODT | amphetamine sulfate | TABLET, ORALLY DISINTEGRATING;ORAL | 209905-004 | Jan 30, 2019 | 10,130,580 | ⤷ Try a Trial |
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-002 | Feb 25, 2011 | 5,583,141 | ⤷ Try a Trial |
| Azurity | EVEKEO ODT | amphetamine sulfate | TABLET, ORALLY DISINTEGRATING;ORAL | 209905-005 | Apr 16, 2021 | 10,130,580 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for AZURITY drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 1 mg/mL | ➤ Subscribe | 2018-08-31 |
International Patents for Azurity Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Mexico | 2009010167 | ⤷ Try a Trial |
| Morocco | 28478 | ⤷ Try a Trial |
| European Patent Office | 1404310 | ⤷ Try a Trial |
| Brazil | PI0414481 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Azurity Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0138441 | SPC/GB95/028 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TOPIRAMATE; REGISTERED: UK 0242/0301 19950718 |
| 1718641 | SPC/GB12/028 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: AZILSARTAN MEDOXOMIL AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING THE POTASSIUM SALT; REGISTERED: UK EU/1/11/734/001-011 20111209 |
| 1915993 | 92315 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON COMPRENANT ALISKIREN,OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLE,ET AMLODIPINE,OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLE |
| 1718641 | 12C0034 | France | ⤷ Try a Trial | PRODUCT NAME: AZILSARTAN MEDOXOMIL ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/11/735/001 20111207 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.