Am Regent Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and what generic alternatives to AM REGENT drugs are available?
AM REGENT has seventy-seven approved drugs.
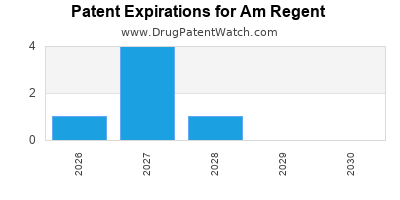
There are seven US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-three supplementary protection certificates in fifteen countries.
Drugs and US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | CUPRIC SULFATE | cupric sulfate | INJECTABLE;INJECTION | 216324-001 | Dec 16, 2022 | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | GEMCITABINE HYDROCHLORIDE | gemcitabine hydrochloride | INJECTABLE;INJECTION | 202031-001 | May 7, 2013 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | LEVOCARNITINE | levocarnitine | INJECTABLE;INJECTION | 075861-001 | Jun 22, 2001 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | ZINC SULFATE | zinc sulfate | SOLUTION;INTRAVENOUS | 209377-002 | Jul 18, 2019 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | TRANEXAMIC ACID | tranexamic acid | INJECTABLE;INJECTION | 201885-001 | Aug 10, 2011 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 10,519,252 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 11,590,097 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,590,097 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-004 | Feb 4, 2022 | 11,123,321 | ⤷ Try a Trial |
| Am Regent | DEXFERRUM | ferric oxyhydroxide | INJECTABLE;INJECTION | 040024-001 | Feb 23, 1996 | 5,624,668 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Am Regent Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Uruguay | 28033 | ⤷ Try a Trial |
| Portugal | 2287204 | ⤷ Try a Trial |
| South Korea | 20180077337 | ⤷ Try a Trial |
| South Korea | 20080082674 | ⤷ Try a Trial |
| Poland | 1973549 | ⤷ Try a Trial |
| Saudi Arabia | 2095 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Am Regent Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0122707 | SPC/GB95/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: GEMCITABINE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY- ACCEPTABLE SALT; REGISTERED: NL RVG17854 19950327; UK 00006/0301 19951026; UK 00006/0302 19951026 |
| 0503785 | 91330 | Luxembourg | ⤷ Try a Trial | CERTIFICATE TITLE: UNE COMBINAISON D'OLMESARTAN MEDOXOMIL, OPTIONNELLEMENT SOUS LA FORME D'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE ET D'HYDROCHLOROTHIAZIDE (OLMETEC PLUS) |
| 1602370 | SPC/GB09/024 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: COMBINATION COMPRISING ALISKIREN, AS THE FREE BASE OR AS A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: CH 5893501 20081028; CH 5893502 20081028; CH 5893503 20081028; CH 5893504 20081028; UK EU/1/08/491/006 20090116; UK EU/1/08/491/002 20090116; UK EU/1/08/491/003 20090116; UK EU/1/08/491/004 20090116; UK EU/1/08/491/005 20090116; UK EU/1/08/491/007 20090116; UK EU/1/08/491/080 20090116; UK EU/1/08/491/074 20090116; UK EU/1/08/491/075 20090116; UK EU/1/08/491/076 20090116; UK EU/1/08/491/077 20090116; UK EU/1/08/491/078 20090116; UK EU/1/08/491/079 20090116; UK EU/1/08/491/068 20090116; UK EU/1/08/4 |
| 0933372 | PA2008006 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: FOSAMPRENAVIR CALCIUM; REGISTRATION NO/DATE: EU/1/04/282/001-002 20040712 |
| 2435024 | 21C1020 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI), GLYCOPYRROLATE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET BUDESONIDE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 0770388 | 2009/012 | Ireland | ⤷ Try a Trial | PRODUCT NAME: QLAIRA-ESTRADIOL VALERATE/DIENOGEST; NAT REGISTRATION NO/DATE: PA1410/58/1 20090109; FIRST REGISTRATION NO/DATE: BE327792 20081103 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.