UTIBRON Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Utibron, and what generic alternatives are available?
Utibron is a drug marketed by Novartis and is included in one NDA. There are three patents protecting this drug.
The generic ingredient in UTIBRON is glycopyrrolate; indacaterol maleate. There are seventeen drug master file entries for this compound. Additional details are available on the glycopyrrolate; indacaterol maleate profile page.
DrugPatentWatch® Generic Entry Outlook for Utibron
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 11, 2028. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for UTIBRON?
- What are the global sales for UTIBRON?
- What is Average Wholesale Price for UTIBRON?
Summary for UTIBRON
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Drug Prices: | Drug price information for UTIBRON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for UTIBRON |
| DailyMed Link: | UTIBRON at DailyMed |
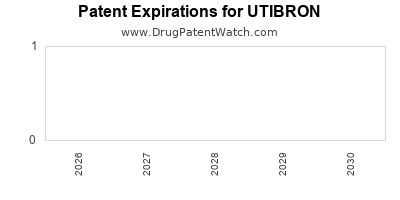
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for UTIBRON
Generic Entry Date for UTIBRON*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
US Patents and Regulatory Information for UTIBRON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | UTIBRON NEOHALER | glycopyrrolate; indacaterol maleate | POWDER;INHALATION | 207930-001 | Oct 29, 2015 | DISCN | Yes | No | 6,878,721 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Novartis | UTIBRON NEOHALER | glycopyrrolate; indacaterol maleate | POWDER;INHALATION | 207930-001 | Oct 29, 2015 | DISCN | Yes | No | 8,182,838 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | UTIBRON NEOHALER | glycopyrrolate; indacaterol maleate | POWDER;INHALATION | 207930-001 | Oct 29, 2015 | DISCN | Yes | No | 8,479,730 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
