PONVORY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ponvory, and when can generic versions of Ponvory launch?
Ponvory is a drug marketed by Vanda Pharms Inc and is included in one NDA. There are six patents protecting this drug.
This drug has one hundred and fifty-one patent family members in forty-two countries.
The generic ingredient in PONVORY is ponesimod. Two suppliers are listed for this compound. Additional details are available on the ponesimod profile page.
DrugPatentWatch® Generic Entry Outlook for Ponvory
Ponvory was eligible for patent challenges on March 18, 2025.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 6, 2032. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PONVORY?
- What are the global sales for PONVORY?
- What is Average Wholesale Price for PONVORY?
Summary for PONVORY
| International Patents: | 151 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 46 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for PONVORY |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PONVORY |
| What excipients (inactive ingredients) are in PONVORY? | PONVORY excipients list |
| DailyMed Link: | PONVORY at DailyMed |
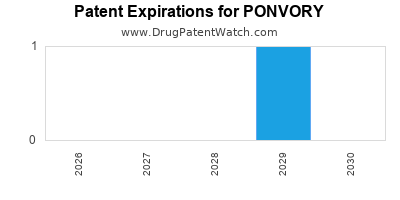

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PONVORY
Generic Entry Date for PONVORY*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PONVORY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Janssen Pharmaceutica N.V., Belgium | Phase 1 |
Pharmacology for PONVORY
| Drug Class | Sphingosine 1-phosphate Receptor Modulator |
| Mechanism of Action | Sphingosine 1-Phosphate Receptor Modulators |
US Patents and Regulatory Information for PONVORY
PONVORY is protected by six US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PONVORY is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for PONVORY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-008 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-004 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-010 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-006 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-010 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-001 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| Vanda Pharms Inc | PONVORY | ponesimod | TABLET;ORAL | 213498-002 | Mar 18, 2021 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for PONVORY
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International N.V. | Ponvory | ponesimod | EMEA/H/C/005163Ponvory is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features. | Authorised | no | no | no | 2021-05-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PONVORY
When does loss-of-exclusivity occur for PONVORY?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3904
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09305980
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0919673
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 40313
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11000867
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2177144
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150391
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16118
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 44465
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 44465
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 59624
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2351
Patent: צורות גבישיות שאינן היגרוסקופיות של (r)-5-[3-כלורו-4-(3,2-דיהידרוקסי-פרופוקסי)-בנז [z]ילאידן]-2-([z]-פרופילאימינו)-3-o-טוליל-תיאזולידין-4-און, המכילות בין 0 ל 0.5 אקוויולנט מים לאקוויוולנט מולקולה, תכשירי רוקחות ושימושים שלהן (Non-hygroscopic crystalline forms of (r)-5-[3-chloro-4-(2,3-dihydroxy-propoxy)-benz[z]ylidene]-2-([z]-propylimino)-3-o-tolyl-thiazolidin-4-one, containing from 0 to 0.5 equivalents of water per equivalent of compound, pharmaceutical compositions and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 08777
Estimated Expiration: ⤷ Get Started Free
Patent: 12505873
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0703
Patent: CRYSTALLINE FORMS OF (R) -5- [3-CHLORO-4-(2,3-DIHYDROXY) - BENZ [Z] YLIDENE] -2- ([Z]-PROPYLIMINO) -3-0-TOLYL-THIAZOLIDIN-4-ONE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11003988
Patent: FORMAS CRISTALINAS DE (R)-5-[3-CLORO-4-(2,3-DIHIDROXI-PROPOXI)-BEN Z[Z]ILIDENO]-2-([Z]-PROPILIMINO)-3-O-TOLIL-TIAZOLIDIN-4-ONA. (CRYSTALLINE FORMS OF (R) -5- [3-CHLORO-4- ( 2, 3-DIHYDROXY-PROPOXY) -BENZ [Z] YLIDENE] -2- ( [Z] -PROPYLIMINO) -3-0-TOLYL-THIAZOLIDIN-4-ONE.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 797
Patent: أشكال البلورية (r) -5 [3 كلورو-4- (2،3-ثنائي هيدروكسي بروبوكسي) بنز [z]-إيليدين] -2 - ([z]-بروبيليمينو)-3-o -طوليل-تيازولين-4-أون
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2854
Patent: CRYSTALLINE FORMS OF (R)-5-[3-CHLORO-4-(2,3-DIHYDROXY-PROPOXY)-BENZ[Z]YLIDENE]-2-([Z]-PROPYLIMINO)-3-O-TOLYL-THIAZOLIDIN-4-ONE
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 44465
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 44465
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 19548
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ (R)-5-[3-ХЛОР-4-(2, 3-ДИГИДРОКСИПРОПОКСИ)БЕНЗ[Z]ИЛИДЕН]-2-([Z]-ПРОПИЛИМИНО)-3-о-ТОЛИЛТИАЗОЛИДИН-4-ОНА (CRYSTALLINE FORMS OF (R)-5-[3-CHLORO-4-(2, 3-DIHYDROXYPROPOXY)BEZ[Z]ILIDEN]-2-([Z]-PROPYLIMINO)-3-o-TOLYLTHIAZOLIDIN-4-ONE)
Estimated Expiration: ⤷ Get Started Free
Patent: 11119898
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ (R)-5-[3-ХЛОР-4-(2,3-ДИГИДРОКСИПРОПОКСИ)БЕНЗ[Z]ИЛИДЕН]-2-([Z]-ПРОПИЛИМИНО)-3-О-ТОЛИЛТИАЗОЛИДИН-4-ОНА
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 44465
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1103691
Patent: CRYSTALLINE FORMS OF (R)-5-[3-CHLORO-4-(2,3-DIHYDROXY-PROPOXY)-BENZ[Z]YLIDENE]-2-([Z]-PROPYLIMINO)-3-0-TOLYL-THIAZOLIDIN-4-ONE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1409597
Estimated Expiration: ⤷ Get Started Free
Patent: 110071133
Patent: CRYSTALLINE FORMS OF (R)-5-[3-CHLORO-4-(2,3-DIHYDROXY-PROPOXY)-BENZ[Z]YLIDENE]-2-([Z]-PROPYLIMINO)-3-0-TOLYL-THIAZOLIDIN-4-ONE
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 34333
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 62911
Estimated Expiration: ⤷ Get Started Free
Patent: 1022220
Patent: Crystalline forms
Estimated Expiration: ⤷ Get Started Free
United Kingdom
Patent: 19182
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PONVORY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 3256125 | ⤷ Get Started Free | |
| China | 106999462 | ⤷ Get Started Free | |
| European Patent Office | 1689726 | DERIVES DE 5-(BENZ-(Z)-YLIDENE)-THIAZOLIDINE-4-ONE UTILISES COMME AGENT IMMUNODEPRESSEURS (5-(BENZ- (Z) -YLIDENE) -THIAZOLIDIN-4-ONE DERIVATIVES AS IMMUNOSUPPRESSANT AGENTS) | ⤷ Get Started Free |
| Japan | 2007511563 | ⤷ Get Started Free | |
| China | 102177144 | ⤷ Get Started Free | |
| South Korea | 20190077131 | ⤷ Get Started Free | |
| Saudi Arabia | 517381489 | توليفة صيدلانية تشتمل على عامل مساعد لمستقبل اس1بي1 انتقائي (PHARMACEUTICAL COMBINATION COMPRISING A SELECTIVE S1P1 RECEPTOR AGONIST) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PONVORY
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3256125 | LUC00262 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: PONESIMOD (NOM IUPAC : (R)-5-(3-CHLORO-4-(2,3-DIHYDROXY-PROPOXY)-BENZ(Z)YLIDENE)-2-((Z)-PROPYLIMINO)-3-O-TOLYL-THIAZOLIDIN-4-ONE), ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; AUTHORISATION NUMBER AND DATE: EU/1/21/1550 20210521 |
| 3256125 | PA2022505,C3256125 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: PONEZIMODAS; REGISTRATION NO/DATE: EU/1/21/1550 20210519 |
| 3256125 | 301174 | Netherlands | ⤷ Get Started Free | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT |
| 3256125 | 2290019-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: PONESIMOD AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; NAT. REG. NO/DATE: EU/1/21/1550 20210521; FIRST REG.: GE EU/1/21/1550 20210521 |
| 3256125 | SPC/GB22/026 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PONESIMOD ((R)-5-(3-CHLORO-4-(2,3-DIHYDROXY-PROPOXY)-BENZ(Z)YLIDENE)-2-((Z)-PROPYLIMINO)-3-O-TOLYL-THIAZOLIDIN-4-ONE) AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU1/21/1550 (FOR NI) 20210521; UK FURTHER MAS ON IPSUM 20210521 |
| 3256125 | C03256125/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: ACTELION PHARMACEUTICALS LTD, CH |
| 3256125 | C202230019 | Spain | ⤷ Get Started Free | PRODUCT NAME: PONESIMOD: (R)-5-(3-CLORO-4-(2,3-DIHIDROXIPROPOXI)-BENZO(Z)IDEN)-2-((Z)-PROPILIMINO)-3-O-TOLIL-TIAZOLIDIN-4-ONA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO.; NATIONAL AUTHORISATION NUMBER: EU/1/21/1550; DATE OF AUTHORISATION: 20210519; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/21/1550; DATE OF FIRST AUTHORISATION IN EEA: 20210519 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for PONVORY
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
