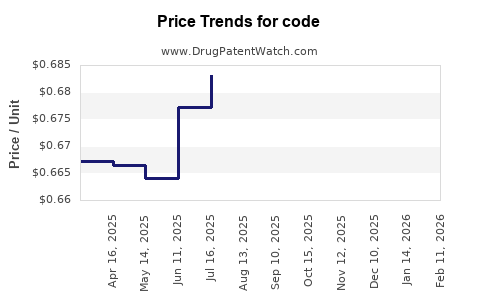
Code - Generic Drug Details
✉ Email this page to a colleague
US Patents and Regulatory Information for code
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hikma | CODEINE SULFATE | codeine sulfate | TABLET;ORAL | 022402-002 | Jul 16, 2009 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Cenci | PROMETHAZINE VC W/ CODEINE | codeine phosphate; phenylephrine hydrochloride; promethazine hydrochloride | SYRUP;ORAL | 088816-001 | Nov 22, 1985 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Amneal Pharms | PROMETHAZINE HYDROCHLORIDE,PHENYLEPHRINE HYDROCHLORIDE W/CODEINE PHOSPHATE | codeine phosphate; phenylephrine hydrochloride; promethazine hydrochloride | SYRUP;ORAL | 200963-001 | Aug 26, 2015 | AA | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |