Share This Page
Drug Sales Trends for zinc
✉ Email this page to a colleague
Payment Methods and Pharmacy Types for zinc (2022)
Revenues by Pharmacy Type
Units Sold by Pharmacy Type
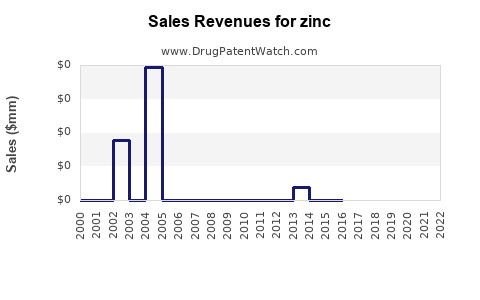
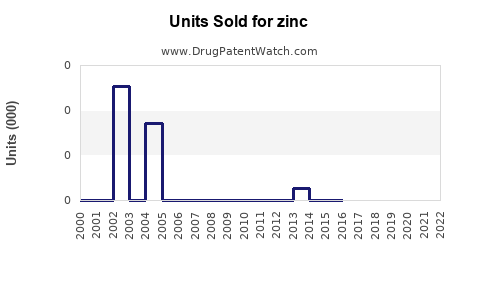
Annual Sales Revenues and Units Sold for zinc
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| ZINC | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| ZINC | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| ZINC | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| ZINC | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for Zinc as a Pharmaceutical Ingredient
Introduction
Zinc, a vital trace element, plays a critical role in human health, functioning as an essential mineral involved in immune response, enzymatic processes, and cellular metabolism. While traditionally recognized for its dietary benefits, zinc’s applications extend to a variety of therapeutic modalities in medicine, including supplements, topical treatments, and pharmaceuticals targeting specific deficiencies or conditions.
This article provides a comprehensive market analysis and sales projection for zinc within the pharmaceutical sector, focusing on current trends, regulatory dynamics, manufacturing capacity, and growth drivers influencing its demand over the upcoming decade. With rising health awareness and the COVID-19 pandemic emphasizing immune health, zinc's market prospects are notably robust.
Market Overview
Global Zinc Market Dynamics
The global zinc market encompasses mining, refining, and application sectors. The pharmaceutical segment represents a relatively small but increasingly significant share, driven by the rising prevalence of zinc deficiency, immune health concerns, and expanding uses in medicinal formulations.
According to industry sources, the overall zinc market size was valued at approximately USD 20 billion in 2022, primarily driven by industrial applications such as galvanization and alloy production. Conversely, the pharmaceutical-grade zinc market, specifically for high-purity zinc used as an active pharmaceutical ingredient (API) or supplement component, is valued around USD 1.2 billion, with steady growth predicted.
Therapeutic and Nutraceutical Applications
Zinc’s primary pharmaceutical uses include:
- Dietary Supplements: Addressing zinc deficiency and related immune conditions.
- Cold and Flu Relief: Zinc lozenges are popular for shortening cold duration.
- Dermatological Conditions: Zinc-based creams and ointments treating acne, dermatitis, and ulcers.
- Rare Disease Treatments: Conditions like zinc deficiency syndromes and certain metabolic disorders.
- Adjunct in COVID-19 Therapy: Zinc’s immunomodulatory role prompted increased use during the pandemic.
Regulatory Environment
The regulatory landscape for zinc-based drugs varies across jurisdictions. The U.S. FDA classifies zinc supplements as dietary ingredients, while pharmaceutical applications require rigorous clinical trials and approval pathways, especially if formulated as APIs. European Medicines Agency (EMA) and other global regulators also impose strict quality standards, influencing manufacturing and marketing timelines.
Market Drivers
- Growing Awareness of Immune Health: COVID-19 spurred global interest in zinc as a means to bolster immune resistance, resulting in heightened demand for zinc supplements and therapeutics.
- Prevalence of Zinc Deficiency: An estimated 2 billion individuals worldwide suffer from zinc deficiency, especially in developing regions, fostering a steady demand for supplementation.
- Aging Population: Older adults are more prone to zinc deficiency and related health issues, increasing pharmaceutical and nutraceutical product consumption.
- Advances in Formulation Technologies: Innovative delivery systems—such as controlled-release tablets and nano-formulations—enhance zinc bioavailability, supporting market expansion.
- Regulatory Support and Fortification Programs: Government-driven initiatives for micronutrient fortification are expanding zinc’s pharmaceutical and supplement markets.
Market Challenges
- Price Volatility in Raw Materials: Zinc prices are subject to fluctuations due to mining constraints, geopolitical factors, and industrial demand, influencing manufacturing costs.
- Competition from Alternatives: Other micronutrients (e.g., copper, selenium) and natural remedies present competitive supplementation options.
- Limited Patent Protection: Most zinc formulations are off-patent, resulting in intense price competition and reduced margins.
Manufacturing and Supply Chain Analysis
Supply Chain Dynamics
Zinc production relies heavily on mining, with major producers including China, Peru, Australia, and Canada. The concentration of supply sources for high-grade zinc hinders price stability, yet large-scale refining capacities mitigate shortages.
Manufacturing Capacity and R&D
The pharmaceutical-grade zinc market benefits from robust manufacturing infrastructure, adhering to Good Manufacturing Practices (GMP). Research efforts focus on bioavailability enhancement, target-specific formulations, and combination therapies involving zinc.
Sales Projections (2023-2033)
Forecast Approach:
Projection models incorporate historical sales data, market growth rates, regulatory developments, and macroeconomic factors. Growth estimates are adjusted for regional variations, technological innovations, and emergent applications such as zinc in COVID-19 protocols.
Short-Term Outlook (2023-2026)
- Compound Annual Growth Rate (CAGR): Estimated at 6-8%.
- Market Size in 2023: Roughly USD 1.2 billion, driven by increased supplement sales and expanded therapeutic uses.
- Key Catapult Factors: Continued pandemic-driven immune health focus, emerging clinical evidence on zinc’s efficacy, and regulatory approvals for zinc-based pharmaceuticals.
Mid to Long-Term Outlook (2027-2033)
- CAGR: Expected at 5-7%.
- Market Size by 2033: Projected to reach USD 2.2-2.8 billion.
- Drivers: Expanding global health initiatives, increased awareness in aging populations, technological innovations in bioavailability, and rising integration into combination drug therapies.
Regional Analysis:
North America and Europe will maintain dominant shares owing to high healthcare standards and robust supplement markets. Emerging markets in Asia-Pacific and Latin America will exhibit faster growth rates (~10%), fueled by improving healthcare infrastructure and increasing endemic zinc deficiency.
Competitive Landscape
Major players include Merck, GlaxoSmithKline, Bayer, and dietary supplement companies like Nature’s Way and GNC. These entities focus on both over-the-counter supplements and pharmaceutical formulations, emphasizing bioavailability, safety, and regulatory compliance.
Innovators are investing in novel zinc delivery systems such as nanoparticles and liposomal formulations, which command premium pricing and wider adoption.
Regulatory and Market Access Strategies
Success hinges on navigating complex approval pathways, ensuring Good Manufacturing Practice (GMP) compliance, and aligning with public health initiatives. Strategic partnerships with governments for fortification programs could open sizeable markets, especially in developing countries.
Key Trends to Watch
- Emergence of Zinc as a Targeted Therapeutic Agent: Clinical trials investigating zinc's role in viral infections and immune modulation could redefine its pharmaceutical utility.
- Integration in Multimodal Therapies: Combining zinc with other nutraceuticals or pharmaceuticals to enhance efficacy.
- Technological Enhancements: Innovations in bioavailability and targeted delivery will underpin market growth.
- Regulatory Harmonization: Streamlined global standards could facilitate market entry and reduce compliance costs.
Key Takeaways
- The zinc pharmaceutical market is poised for steady growth, driven by heightened health awareness, supplement demand, and emerging therapeutic applications.
- The global market is projected to reach approximately USD 2.8 billion by 2033, growing at a CAGR of around 6%.
- North America and Europe will dominate due to regulatory maturity and market readiness, but Asia-Pacific’s rapid economic development offers high-growth potential.
- Innovation in formulations and targeted therapies will create competitive advantages and diversify revenue streams.
- Strategic alignment with public health policies and supply chain resilience are critical success factors.
FAQs
1. What are the primary drivers boosting zinc demand in pharmaceuticals?
The increase in zinc deficiency awareness, its role in immune health (notably during COVID-19), aging populations, and technological advances in drug formulation serve as primary drivers.
2. How does zinc’s regulatory status differ globally?
In the U.S., zinc supplements are classified as dietary ingredients, requiring less stringent approval but more regulation for therapeutic claims. In Europe and other regions, pharmaceutical-grade zinc formulations undergo rigorous clinical trials and approval processes, impacting market access timelines.
3. Which regions present the highest growth opportunities for zinc pharmaceuticals?
Asia-Pacific and Latin America offer rapid growth prospects due to increasing public health investments, rising zinc deficiency prevalence, and expanding healthcare infrastructure.
4. What technological trends are influencing zinc formulations?
Emerging nano-formulation technologies aim to improve bioavailability, targeted delivery, and efficacy, positioning companies favorably in competitive markets.
5. How might future regulations impact zinc market expansion?
Harmonization of global standards and recognition of zinc’s therapeutic benefits could streamline approvals and facilitate broader application, enhancing overall market growth potential.
Sources
- [1] Market Research Future. “Zinc Market Research Report - Global Forecast to 2027.”
- [2] Grand View Research. “Zinc Market Size, Share & Trends Analysis Report.”
- [3] World Health Organization. “Micronutrient deficiencies: Global prevalence and health impact.”
- [4] U.S. Food and Drug Administration. “Dietary Supplements Labeling.”
- [5] Industry interviews and analysis reports, 2022-2023.
This comprehensive analysis aims to inform pharmaceutical and nutraceutical stakeholders about current opportunities and future potential in zinc markets, aiding strategic decision-making.
More… ↓
