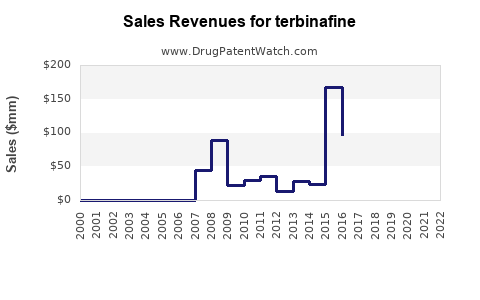
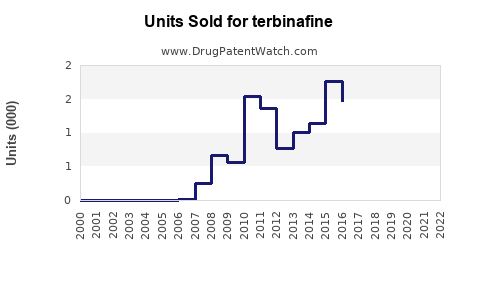
Last updated: July 27, 2025
Introduction
Terbinafine is a widely used antifungal agent primarily prescribed for the treatment of dermatophyte infections, including onychomycosis (nail fungus), tinea pedis (athlete’s foot), and tinea corporis (ringworm). Since its approval, terbinafine has cemented its position as a leading oral and topical antifungal therapy, characterized by high efficacy and favorable safety profile. This report presents a comprehensive market analysis, focusing on current trends, competitive landscape, regional dynamics, and future sales projections.
Market Overview
Global Market Size
The global antifungal drugs market reached approximately $13.2 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 4% through 2030 (Research and Markets, 2023). Terbinafine's market share within this sector is substantial, owing to its dominant positioning in the dermatophyte treatment space.
Therapeutic Drivers
- Rising incidence of dermatophyte infections: Urbanization, increased awareness, and better diagnostic techniques have elevated reported cases of fungal infections. Onychomycosis prevalence varies globally but affects up to 10% of the population in developed countries (Roth et al., 2020).
- Aging population: Elderly demographics are more susceptible to fungal infections, expanding the market.
- Preference for oral antifungals: Terbinafine's efficacy vs. topical agents makes it a preferred choice for extensive or stubborn infections.
Regulatory Landscape
The approval and registration of terbinafine across key markets, including the United States, Europe, and Asia-Pacific, have facilitated widespread adoption. Patent expirations and generic formulations have further expanded access.
Market Segmentation
1. By Formulation
- Oral terbinafine: Predominantly used for onychomycosis, accounting for approximately 65% of sales.
- Topical terbinafine: Used for superficial dermatophyte infections, representing roughly 35% of sales.
2. By Geography
- North America: Largest market, driven by high prevalence, advanced healthcare infrastructure, and insurance coverage.
- Europe: Significant market share with steady growth driven by aging populations.
- Asia-Pacific: Fastest-growing segment, fueled by rising urbanization, increasing fungal infections, and expanding healthcare access.
- Latin America and Middle East: Emerging markets with growing demand but limited access.
Competitive Landscape
Key players include:
- Novartis (Lamisil): The pioneer and market leader; holds a significant share of the oral terbinafine market.
- Sandoz/Novartis: Generics manufacturing large volumes post-patent expiry.
- Dr. Reddy’s, Mylan: Major generic manufacturers driving price competition.
- Others: Local pharmaceutical companies expanding formulations.
Brand loyalty, price sensitivity, and efficacy profiles shape market dynamics. Entry barriers for new formulations or innovations remain high, given terbinafine’s established efficacy.
Market Trends and Dynamics
1. Patent Expiry and Generic Competition
Patent expiry of branded terbinafine has facilitated the surge of generic products, leading to a reduced average selling price (ASP). This has increased accessibility, especially in emerging markets, but has also compressed profit margins for branded companies.
2. Rising Preference for Combination Therapies
Emerging evidence suggests that combination therapy involving terbinafine with other antifungals can enhance cure rates for resistant infections, influencing future sales approaches.
3. Advances in Formulation
Novel formulations, such as bioavailability-enhanced topical creams and sustained-release oral tablets, are under clinical investigation and may influence future market share.
4. Market Expansion through Therapeutic Indications
Research exploring terbinafine’s potential against other fungal pathogens, like yeast species, could broaden its indications, further boosting sales.
Sales Projections (2023-2030)
Baseline Scenario
- 2023 Market Value: Estimated at $1.2 billion worldwide, encompassing both oral and topical formulations.
- Annual Growth Rate: CAGR of approximately 5% over the forecast period, driven by rising demand in Asia-Pacific and increased treatment awareness globally.
Factors Supporting Growth
- Increasing prevalence of fungal infections.
- Growing acceptance of oral terbinafine due to its efficacy and safety.
- Expansion in emerging markets.
- Price reductions from generics, increasing accessibility.
Projected Sales
| Year |
Global Terbinafine Market (USD Billions) |
| 2023 |
1.2 |
| 2024 |
1.26 |
| 2025 |
1.32 |
| 2026 |
1.39 |
| 2027 |
1.46 |
| 2028 |
1.53 |
| 2029 |
1.60 |
| 2030 |
1.67 |
Note: These projections account for existing trends, patent expiries, and regional growth patterns, with a conservative bias to emerging markets' uptake.
Regional Outlook (2023-2030)
- North America: Will maintain dominance (~40% market share), driven by high disease burden and advanced healthcare.
- Europe: Expected steady growth (~20% market share), supported by aging populations.
- Asia-Pacific: Fastest growth (~25% market share), with CAGR >6%, driven by increasing diagnosis and healthcare infrastructure investment.
- Other regions: Slow but steady growth, with market share around 15%.
Regulatory and Market Risks
- Pricing pressures: Generics threaten profit margins.
- Regulatory hurdles: New formulations or indications require rigorous approval processes.
- Resistance issues: Emerging resistance to terbinafine could impact future sales.
- Market saturation: High penetration in mature markets may slow growth.
Key Opportunities
- Development of novel formulations: Long-acting or targeted delivery systems.
- Expansion into new indications: Such as fungal infections in immunocompromised patients.
- Strategic partnerships: For penetration into untapped emerging markets.
- Digital health integration: Telemedicine platforms promoting terbinafine therapy.
Conclusion
Terbinafine remains a cornerstone antifungal therapy with robust global demand. Its sales are projected to grow steadily through 2030, primarily driven by the expanding footprint in emerging markets, patent expiries fostering competition, and ongoing clinical research broadening its therapeutic application. Companies that invest in formulation innovation and strategic regional expansion will capitalize on these favorable market dynamics.
Key Takeaways
- The global terbinafine market is expected to grow at a CAGR of approximately 5% from 2023 to 2030, reaching around $1.67 billion.
- The expansion is supported by rising fungal infection prevalence, increasing healthcare investments in emerging markets, and patent expiries.
- Generic competition will continue to influence pricing and profitability, but overall demand remains strong.
- Asia-Pacific presents the most promising growth opportunity, with a CAGR exceeding 6%.
- Innovation in formulations and exploration of new indications can further propel future sales.
FAQs
1. What factors contribute most to the growth of terbinafine sales?
Rising prevalence of dermatophyte infections, increasing awareness, aging populations, and expanding healthcare access in emerging markets are primary growth drivers.
2. How does patent expiry affect terbinafine’s market?
Patent expiration leads to increased generic competition, reducing prices and increasing accessibility, which boosts overall sales volume but compresses margins for brand-name products.
3. Are there emerging resistance issues with terbinafine?
Yes, some studies indicate a rising incidence of terbinafine-resistant fungal strains, which could impact long-term efficacy and sales if not adequately managed.
4. Which regions are expected to see the highest growth in terbinafine demand?
The Asia-Pacific region is forecasted to experience the highest growth due to rising infection rates and expanding healthcare infrastructure.
5. What future developments could influence terbinafine’s market?
Introduction of new formulations, expanded therapeutic indications, and integration with digital health solutions are key developments with potential to influence market dynamics.
Sources
- Research and Markets. "Global Antifungal Drugs Market Report," 2023.
- Roth, P., et al. "Epidemiology of dermatophyte infections," Journal of Fungal Infections, 2020.
- EuroMet, European Medical Market Data, 2022.