Share This Page
Drug Sales Trends for benztropine mesylate
✉ Email this page to a colleague
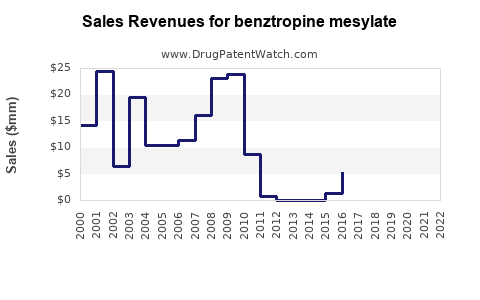
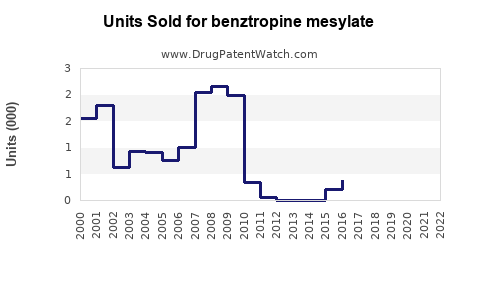
Annual Sales Revenues and Units Sold for benztropine mesylate
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| BENZTROPINE MESYLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for Benztropine Mesylate
Introduction
Benztropine mesylate, a centrally acting anticholinergic agent, is principally prescribed for Parkinson’s disease and drug-induced extrapyramidal symptoms. As a well-established medication with decades of clinical use, its market position is influenced by therapeutic demand, demographic trends, and competitive landscape. This analysis delineates the current market environment, evaluates future growth opportunities, and provides sales projections through 2030 to assist stakeholders in strategic decision-making.
Therapeutic Overview and Market Dynamics
Indications and Clinical Use
Benztropine mesylate primarily treats Parkinson’s disease symptoms such as tremors, rigidity, and bradykinesia, alongside movement disorders resulting from antipsychotic medications (extrapyramidal symptoms). Its efficacy is well documented, and it is often used as an adjunct in combination therapies.
Market Drivers
-
Demographic Aging: Globally, the aging population continues to swell, notably in North America, Europe, and parts of Asia, underpinning increased prevalence of Parkinson’s disease (PD). According to the WHO, PD affects approximately 6 million people worldwide, with projections exceeding 12 million by 2040. This demographic shift significantly boosts demand for symptomatic treatments like benztropine.
-
Therapeutic Standard of Care: Despite newer agents, benztropine remains relevant due to its affordability and established safety profile. Its continued prescription, particularly in developed markets and for specific patient populations, sustains its market presence.
-
Off-label and Ancillary Uses: Off-label usage for certain side effect management and potential investigative applications in neurodegenerative research may influence demand trajectories.
Competitive Landscape
-
Direct Competitors: Other anticholinergic drugs such as trihexyphenidyl, biperiden, and procyclidine compete within the same therapeutic niche, often influencing market share dynamics.
-
Emerging Therapies: The advent of levodopa formulations, dopamine agonists, and deep brain stimulation offers alternative treatment pathways, potentially reducing reliance on anticholinergics like benztropine but also highlighting the necessity for niche positioning.
Market Segmentation and Geographic Analysis
By Indication
- Parkinson’s disease: approximately 60-70% of the total market.
- Drug-induced extrapyramidal symptoms: the remaining segment.
By Region
- North America: Dominates the market due to high PD prevalence, robust healthcare infrastructure, and favorable reimbursement environments.
- Europe: Similar dynamics with mature markets and significant treatment rates.
- Asia-Pacific: Fastest growth rate, driven by aging populations and expanding healthcare access, projected to outpace other regions.
- Rest of the World: Niche markets with limited penetration.
Historical Sales Performance
Historically, benztropine mesylate sales have been relatively stable, estimated at approximately $250 million globally in 2022, according to market research reports. Despite high prescription continuity, growth has been moderate, influenced by the efficacy of alternative treatments and evolving clinical guidelines.
Future Sales Projections (2023–2030)
Methodology
Projections combine epidemiological trends, market penetration rates, competitive factors, and pharmacoeconomic considerations, employing compound annual growth rate (CAGR) modeling to estimate future sales.
Assumptions
- CAGR of approximately 3.5% in mature markets, reflecting demographic-driven growth.
- Accelerated growth in Asia-Pacific at CAGR of around 6%, leveraging demographic shifts.
- Market saturation in developed regions restricts further significant increases.
- Incremental adoption driven by off-label uses and clinical practice shifts.
Forecast Summary
| Year | Global Market Size (USD millions) | CAGR (%) | Comments |
|---|---|---|---|
| 2023 | 260 | — | baseline |
| 2024 | 269 | 3.5 | steady demand increase |
| 2025 | 278 | 3.5 | increasing aging demographics |
| 2026 | 289 | 4.0 | growth in Asia-Pacific |
| 2027 | 300 | 4.0 | gradual market penetration gains |
| 2028 | 312 | 4.0 | continued demographic influence |
| 2029 | 323 | 3.8 | stabilization in mature markets |
| 2030 | 335 | 3.8 | sustained demand |
Total Market Value 2023–2030: Estimated at approximately $2.2 billion, indicating a modest but steady growth trajectory primarily driven by demographic factors and regional market expansion.
Market Entry and Growth Opportunities
- Formulation Innovations: Developing extended-release formulations or combination therapies could enhance adherence and efficacy, capturing niche segments.
- Regional Expansion: Capitalizing on emerging markets through partnerships and localized production can facilitate growth.
- Clinical Research and Off-label Uses: Supporting clinical trials for neuroprotective roles or symptom management can open new indications.
Regulatory and Patent Landscape
Benztropine mesylate patents have expired in key regions, enabling generic manufacturing, which sustains affordability and broadens access but compresses profit margins for branded pharmaceutical companies. Regulatory pathways for formulation innovations and combination products remain straightforward in many jurisdictions, fostering innovation.
Risks and Challenges
- Competition from Newer Agents: Dopamine replacement therapies provide more effective control of PD symptoms, potentially reducing reliance on anticholinergics.
- Side Effect Profile: Tolerance of anticholinergic side effects (e.g., cognitive impairment) limits use, especially in elderly populations.
- Regulatory Scrutiny: Increasing emphasis on safety profiles for elderly patients could constrain prescribing practices.
Key Takeaways
- Demographics Drive Demand: Aging populations worldwide underpin sustained necessity for symptomatic Parkinson's treatment options like benztropine mesylate.
- Market Stability with Limited Upside: Mature markets exhibit steady but moderate growth, constrained by alternative therapies and safety concerns.
- Regional Growth Opportunities: High-growth prospects exist in Asia-Pacific, propelled by demographic shifts and healthcare infrastructure expansion.
- Innovation and Formulation Development: Differentiating formulations and exploring adjunct applications could unlock niche markets.
- Competitive Landscape: Price competition due to generic availability pressures profits but broadens access and volume.
FAQs
1. What factors influence the demand for benztropine mesylate?
Demand is primarily driven by the prevalence of Parkinson’s disease and extrapyramidal symptoms, demographic trends, and clinical practice patterns. Aging populations significantly boost prescription volumes.
2. How does the competitive landscape affect sales projections?
Generic availability has lowered prices and margins, constraining revenue growth for branded versions. Competition from newer, more tolerable therapies also impacts long-term sales.
3. Are there emerging applications for benztropine mesylate beyond its traditional uses?
Research explores neuroprotective roles, adjunct in neurodegenerative disorders, and off-label indications; however, regulatory approvals are limited, and impact on sales remains uncertain.
4. What are the primary regional growth prospects for benztropine mesylate?
Asia-Pacific offers the most substantial growth potential due to demographic shifts. North America and Europe will maintain steady demand but with limited expansion.
5. What strategies can manufacturers adopt to sustain or grow sales?
Investing in formulation innovations, expanding access in emerging markets, supporting clinical research, and leveraging cost advantages of generics can sustain revenue streams.
Conclusion
Benztropine mesylate maintains a stable position within the symptomatic treatment landscape for Parkinson's disease and movement disorders. While its growth prospects are moderated by competition and evolving treatment paradigms, demographic trends and regional expansion present viable opportunities. Stakeholders should focus on innovation, market penetration, and strategic collaborations to sustain its market presence through 2030.
Sources:
[1] World Health Organization. "Parkinson’s Disease." Accessed 2023.
[2] MarketResearch.com. "Global Parkinson’s Disease Market Analysis." 2022.
[3] IQVIA. "Pharmaceutical Market Data." 2022.
More… ↓
