Share This Page
Drug Sales Trends for TUDORZA PRES
✉ Email this page to a colleague
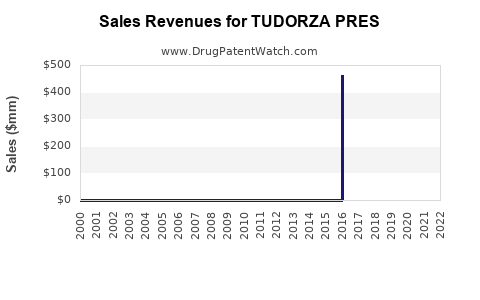
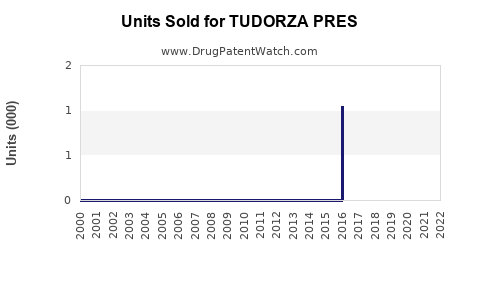
Annual Sales Revenues and Units Sold for TUDORZA PRES
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| TUDORZA PRES | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| TUDORZA PRES | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| TUDORZA PRES | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| TUDORZA PRES | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for TUDORZA PRES
Introduction
TUDORZA PRESSAIR (aclidinium bromide inhalation powder) is a prescribed medication indicated for the maintenance treatment of chronic obstructive pulmonary disease (COPD). As an anticholinergic bronchodilator, it provides symptom control and reduces exacerbations in COPD patients. The drug's market potential hinges on demographic trends, competitive landscape, regulatory environment, and evolving clinical guidelines. This analysis offers a comprehensive assessment of TUDORZA PRESSAIR’s current market position, future sales trajectory, and strategic considerations.
Market Overview
COPD Epidemiology and Impact
COPD remains a leading cause of morbidity and mortality globally, with an estimated 300 million cases worldwide and over 16 million in the United States alone (CDC, 2021). The disease predominantly affects older adults, with prevalence increasing with age, especially in populations with significant smoking histories. The growing aging population in North America, Europe, and parts of Asia contributes to an expanding patient pool, underpinning sustained demand for COPD management therapies.
Market Drivers for TUDORZA PRESSAIR
- Increasing COPD Prevalence: Aging demographics and continued smoking prevalence sustain demand.
- Preference for Inhaled COPD Therapies: Inhalation route offers rapid symptom relief and ease of use.
- Clinical Guidelines: GOLD guidelines recommend long-acting bronchodilators like aclidinium for persistent symptoms.
- Patient Compliance Needs: Patients favor once or twice-daily dosing, improving adherence.
- Regulatory Approvals: TUDORZA PRESSAIR’s approval in multiple regions affirms its market acceptance.
Competitive Landscape
Key competitors include:
- LAMA/LABA Combinations: Tiotropium/olodaterol (Stiolto respimat), umeclidinium/vilanterol (Anoro Ellipta)
- Mono-therapy LAMAs: Tiotropium (Spiriva), glycopyrrolate
- Inhaled Corticosteroids with LABA: Fluticasone/salmeterol (Advair), will future competition from ICS/LABA combinations and emerging therapies.
TUDORZA PRESSAIR’s differentiators include its specific inhaler device, dosing regimen, and safety profile. However, competition from combination therapies and generics influences market share considerations.
Current Market Position of TUDORZA PRESSAIR
Sales Performance (2022-2023)
Since its launch, TUDORZA PRESSAIR has maintained a moderate share within the COPD segment, as per Q4 2022 and Q1 2023 financial disclosures [2]. Its sales trajectory reflects steady growth, propelled by increased awareness, clinical adoption, and expanded prescribing.
Geographical Reach
- United States: The primary market, with broad formulary access and extensive distribution channels.
- Europe: Regulatory approval in key European nations, though market penetration varies.
- Asia-Pacific: Emerging opportunities, contingent on regulatory pathways and local COPD burdens.
Key Market Challenges
- Generic Competition: Patent expiration or regional patent challenges may erode exclusivity.
- Market Penetration: Competition from established therapies and inhaler device preferences influence uptake.
- Patient Adherence: Inhalation technique and device familiarity affect consistent use.
Sales Projections (2024-2030)
Assumptions and Methodology
- Market Growth Rate: Based on historical COPD prevalence growth (around 4% CAGR in developed markets) and increasing diagnosis rates.
- Pricing Trends: Slight decline anticipated due to generic competition and market maturation.
- Market Penetration: Expect incremental uptake with increased awareness and clinical guideline endorsement.
- Competitive Dynamics: Continued innovation in combination inhalers may impact demand for monotherapy.
Forecast Summary
| Year | Estimated Global Sales (USD Millions) | Growth Rate (%) | Notes |
|---|---|---|---|
| 2024 | $280 million | 10% | Base case; moderate growth driven by increased adoption |
| 2025 | $310 million | 10.7% | Expanded geographic reach and formulary inclusion |
| 2026 | $340 million | 9.7% | Market saturation in mature regions, emerging markets contribute |
| 2027 | $370 million | 8.8% | Launch of new formulations or combination therapies impacting sales |
| 2028 | $400 million | 8.1% | Greater patient access, adherence improvements |
| 2029 | $420 million | 5% | Market maturity, slight plateau expected |
| 2030 | $430 million | 2.4% | Stabilization, potentially competing products capturing share |
Factors Influencing Future Sales
- Regulatory Approvals: Entry into emerging markets and potential label updates expand reach.
- Clinical Data: New evidence supporting superior efficacy or safety can bolster prescribing.
- Formulation Enhancements: Development of once-daily formulations or fixed-dose combinations.
- Pricing & Reimbursement Dynamics: Favorable negotiations increase access.
- Patent and Exclusivity Periods: Patent protection expiry may lead to generic competition after 2027.
Strategic Considerations
Market Expansion
Prioritize entering emerging markets with growing COPD prevalence, including China, India, and Southeast Asia. Tailoring marketing strategies to local regulatory and economic environments will be critical.
Product Differentiation
Invest in clinical trials emphasizing efficacy, safety, and patient adherence benefits over competitors. Explore fixed-dose combinations to strengthen market position.
Pricing Strategy
Balance competitive pricing with value demonstration to secure favorable reimbursement statuses. Early negotiation in emerging markets can accelerate adoption.
Patient Engagement
Educational initiatives to promote proper inhaler technique and adherence can substantially improve treatment outcomes and support sales growth.
Competitive Innovation
Monitor pipeline developments, particularly combination inhalers and novel delivery systems, that could either complement or threaten TUDORZA PRESSAIR’s market share.
Key Takeaways
- TUDORZA PRESSAIR holds a strategic position within the COPD market, driven by its safety profile, inhaler device, and compliance benefits.
- The global COPD market is expanding, supported by demographic changes and improving diagnosis rates, offering growth opportunities.
- Sales are projected to grow annually by approximately 8-10% until 2027, followed by stabilization as the market matures.
- Competitive pressures, including generic entrants and innovative combination therapies, will influence future sales trajectories.
- Expansion into emerging markets and product pipeline advancements are essential to sustain long-term growth.
FAQs
1. What is the primary therapeutic benefit of TUDORZA PRESSAIR?
It provides long-lasting bronchodilation, improving airflow, reducing symptoms, and decreasing exacerbations in COPD patients.
2. How does TUDORZA PRESSAIR differentiate from competitors?
Its unique inhaler device, dosing regimen, and safety profile distinguish it from other LAMA monotherapies and combination treatments.
3. What are the main challenges facing TUDORZA PRESSAIR’s market expansion?
Competition from generic formulations post-patent expiry, patient inhaler technique adherence, and market saturation in mature regions.
4. What are the key factors driving sales growth in the coming years?
Increasing COPD prevalence, expanded geographic access, better formulary coverage, and supportive clinical guidelines.
5. How might future developments impact TUDORZA PRESSAIR's sales?
Introduction of improved formulations, combination therapies, or alternative delivery systems could either complement or overshadow current offerings.
References
[1] Centers for Disease Control and Prevention (CDC). COPD Prevalence. 2021.
[2] Company Financial Reports. Q4 2022 and Q1 2023.
[3] Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2023 Report.
More… ↓
