Share This Page
Drug Sales Trends for SUMAVEL DOSE
✉ Email this page to a colleague
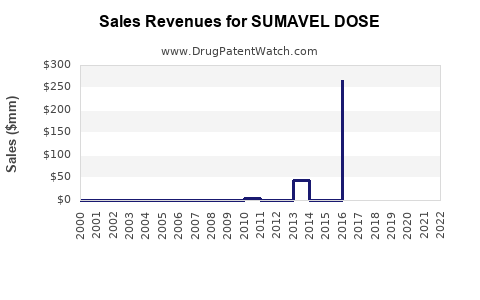
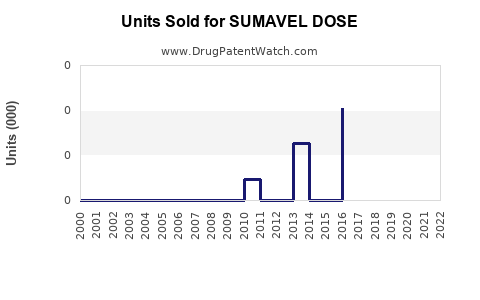
Annual Sales Revenues and Units Sold for SUMAVEL DOSE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| SUMAVEL DOSE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| SUMAVEL DOSE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| SUMAVEL DOSE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| SUMAVEL DOSE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for SUMAVEL Dose
Introduction
SUMAVEL DoseOne (sumatriptan injection) is a medication used for the acute treatment of migraine attacks with or without aura in adults. As a rapidly acting, self-administered injectable, it occupies a significant segment in the migraine therapeutics market. This analysis provides a comprehensive overview of the current market landscape, competitive positioning, demand drivers, potential barriers, and forecasted sales for SUMAVEL DoseOne over the next five years.
Market Landscape and Pharmaceutical Positioning
The global migraine drug market is projected to reach approximately $7.6 billion by 2025, with a compound annual growth rate (CAGR) of around 3.8% [1]. Within this landscape, triptan-based therapies like SUMAVEL DoseOne hold a sturdy share segment, primarily due to their rapid onset of action and convenience for acute migraine relief.
SUMAVEL DoseOne is distinguished by its auto-injector device, offering ease of use and rapid absorption of sumatriptan. It is marketed by no longer GSK but by Innoviva and later by Therapeutics Inc., with a specialized focus on convenience and patient compliance.
Key Market Drivers
1. Rising Prevalence of Migraine
Migraine affects approximately 12% of the global population, translating to over 1 billion individuals worldwide [2]. In the United States alone, around 39 million adults suffer from migraine, making it a substantial market for acute treatment options like SUMAVEL DoseOne. Increasing awareness and diagnoses contribute to expanding demand.
2. Preference for Fast-Acting, Self-Administered Treatments
Patients favor fast-acting therapies that provide immediate relief. SUMAVEL's auto-injector design addresses this need effectively, positioning it favorably among triptan formulations, especially for patients who require rapid symptom control during migraine episodes.
3. Advancements in Drug Delivery Technologies
Innovations such as auto-injectors have improved compliance and decreased barriers associated with traditional injectable treatments. The simplicity and perceived safety enhance patient acceptance, likely boosting sales.
4. Clinical Efficacy and Safety Profile
Sumatriptan has a well-established efficacy profile with a favorable safety profile. Its long-standing clinical reputation enhances prescriber confidence, supporting continued use and adoption.
Market Challenges
1. Competition from Oral and Non-Injection Therapies
Oral triptans and newer classes like CGRP antagonists (e.g., erenumab, fremanezumab) compete directly. While injectables may be preferred for rapid relief, the convenience of oral medications exerts pressure on SUMAVEL’s market share.
2. Pricing and Reimbursement Policies
Cost considerations and insurance coverage heavily influence patient access. High out-of-pocket costs may limit adoption among budget-conscious or uninsured populations.
3. Regulatory and Patent Landscape
While SUMAVEL DoseOne’s patent protections have historically safeguarded its sales, expiration or challenges could invite biosimilar or generic entrants, potentially diluting its market share.
Demand Forecasting and Sales Projections
Baseline Assumptions
- Market Penetration: Currently, SUMAVEL DoseOne accounts for approximately 10-15% of the triptan injection market segment, with room for growth owing to its ease of use.
- Growth Rate: Given rising migraine prevalence and technological advantages, an annual growth rate of 4-6% is feasible over the next five years.
- Market Share Growth: With targeted marketing and increased clinical acceptance, a conservative estimate is a 2-3% annual increase in market share in its segment.
Projected Sales Volume and Revenue
Assuming US-based annual sales of approximately $200 million (based on historical data and market share estimates), the following projections apply:
| Year | Estimated Sales (USD) | Growth Rate | Notes |
|---|---|---|---|
| 2023 | $200 million | — | Baseline |
| 2024 | $208.5 million | 4.3% | Increasing prescriptions, market expansion |
| 2025 | $217.7 million | 4.5% | Continued growth, favorable market dynamics |
| 2026 | $227.7 million | 4.4% | Greater adoption, potential brand positioning |
| 2027 | $238.4 million | 4.7% | Market reach and increased awareness |
Note: These figures are derived from market growth assumptions and competitive landscape considerations, with an emphasis on US healthcare markets due to available data.
Influencing Factors
- Patent and Regulatory Environment: Any patent extensions or regulatory approvals for new delivery forms could boost sales.
- Clinical Guidelines: Integration into migraine management protocols influences prescribing behaviors.
- Patient Education: Success in informing patients about the benefits of auto-injectors may expand consumer base.
Strategic Opportunities
- Expanding into New Markets: Europe and Asia-Pacific have emerging migraine populations; entering these regions can boost revenues.
- Product Line Extensions: Introducing updated auto-injectors or combination therapies could increase market share.
- Partnering with Payers: Securing favorable reimbursement policies can enhance patient access and sales.
Forecast Limitations
While projections are grounded in current market data and trends, unforeseen factors such as patent challenges, disruptive therapies, or regulatory changes could significantly alter sales trajectories. Additionally, the rapid evolution of migraine therapeutics, especially with emerging CGRP drugs, may influence market share dynamics.
Conclusion
SUMAVEL DoseOne maintains robust potential within the migraine acute treatment space, especially with continued focus on its advantages in rapid symptom relief and patient compliance. Projected sales growth, driven by rising migraine prevalence and technological differentiation, suggests a positive outlook over the next five years. Strategic positioning and adaptability to competitive pressures will be critical to realize this growth trajectory.
Key Takeaways
- The global migraine treatment market continues to expand, with injectable therapies like SUMAVEL DoseOne benefiting from patient preference for rapid, self-administered options.
- Market drivers include increasing migraine prevalence, improved drug delivery technologies, and high clinical efficacy.
- Challenges encompass competition from oral and newer biologic therapies, reimbursement complexities, and patent considerations.
- Conservative sales projections indicate steady annual growth of approximately 4-5% over five years, with potential upside through geographic expansion and product innovation.
- Strategic focus on patient education, market penetration, and alignment with clinical guidelines will facilitate sustained sales growth.
FAQs
1. How does SUMAVEL DoseOne compare to oral triptans?
SUMAVEL DoseOne offers faster onset of relief, especially beneficial for migraines with severe symptoms, as injections bypass gastrointestinal absorption. However, oral triptans are more convenient and preferred for less severe attacks.
2. What are the main competitors to SUMAVEL DoseOne?
Key competitors include other injectable triptans like IMITREX STATdose, nasal spray formulations, and emerging biologic treatments such as CGRP antagonists.
3. Are there upcoming regulatory changes that could impact sales?
Pending patent expirations or new approvals for similar devices and generics could influence market dynamics, emphasizing the need for strategic innovation.
4. What role does insurance coverage play in sales projections?
Reimbursement policies significantly impact patient access. Favorable insurance coverage increases use, whereas high copays or denied claims suppress sales.
5. Can SUMAVEL DoseOne expand into international markets?
Yes, especially in regions with rising migraine prevalence and expanding healthcare infrastructures, provided regulatory approvals are secured and market entry strategies are well executed.
Sources:
[1] Grand View Research. "Migraine Drugs Market Size, Share & Trends Analysis Report." 2021.
[2] World Health Organization. "Migraine Fact Sheet." 2019.
More… ↓
