Share This Page
Drug Sales Trends for RESTASIS OP
✉ Email this page to a colleague
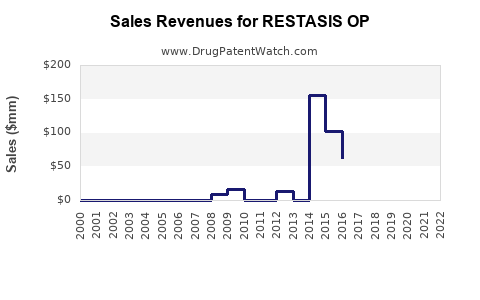
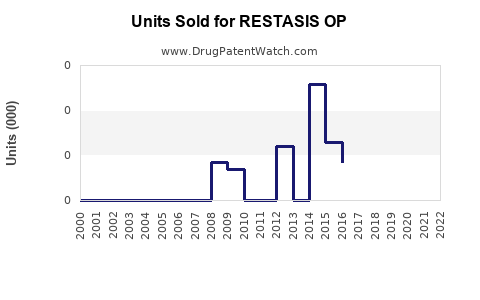
Annual Sales Revenues and Units Sold for RESTASIS OP
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| RESTASIS OP | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| RESTASIS OP | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| RESTASIS OP | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| RESTASIS OP | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| RESTASIS OP | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for RESTASIS OP
Introduction
Restasis OP (ophthalmic), a prescription eye drop formulation developed by Allergan (acquired by AbbVie in 2020), represents an innovative advancement in the treatment of keratoconjunctivitis sicca, commonly known as dry eye disease. With its unique sustained-release delivery system and notable market presence, Restasis OP is positioned for substantial growth amid a competitive ophthalmic therapeutics landscape. This analysis evaluates current market dynamics, potential growth drivers, competitive landscape, regulatory environment, and provides detailed sales projections for the upcoming years.
Market Overview
Dry Eye Disease (DED) Market Landscape
Dry eye disease affects approximately 5-15% of the adult population, with prevalence increasing in aging populations and among contact lens wearers [1]. The global dry eye market was valued at approximately USD 4.4 billion in 2021 and is projected to reach USD 6.5 billion by 2028, registering a Compound Annual Growth Rate (CAGR) of about 5.7% [2].
Restasis OP’s Therapeutic Positioning
Restasis OP delivers cyclosporine A via a novel sustained-release intratympanic implant, offering prolonged therapeutic effects over traditional drops. This formulation aims to improve patient adherence and outcomes, potentially increasing prescription frequency and patient retention.
Market Penetration
Since its launch, Restasis OP has found a niche among ophthalmologists seeking long-acting alternatives to traditional cyclosporine eye drops. However, adoption remains limited relative to competition from newer therapies like lifitegrast (Xiidra) and emerging biologics.
Competitive Landscape
| Product | Type | Mechanism | Market Share (2022) | differentiators |
|---|---|---|---|---|
| Restasis OP | Intratympanic implant | Sustained-release cyclosporine | ~10% | Long duration, improved adherence |
| Xiidra | Eye drops | LFA-1 antagonist | ~40% | Faster onset, established brand recognition |
| Cequa | Eye drops | Cyclosporine A with nanomicellar technology | ~20% | Enhanced bioavailability |
| Emerging biologics | Injectables/Injections | Anti-inflammatory biologics | ~15% | Potential for high efficacy |
Market Differentiation
Restasis OP's sustained-release delivery addresses critical patient compliance issues, a key barrier in ophthalmic therapy adherence. However, higher costs and procedural requirements for implantation limit its market penetration compared to simple eye drops.
Regulatory and Reimbursement Environment
Regulatory Status
- Approved by the FDA in 2021, designed for long-lasting treatment with a single implant every 6-12 months.
- Regulatory considerations focus on procedural safety, device usability, and long-term safety data.
Reimbursement Dynamics
Reimbursement strategies involve coverage under medical and pharmacy benefits, with potential variability based on healthcare systems and insurer policies. The higher upfront procedural cost is offset by reduced patient visits and improved adherence.
Market Drivers and Barriers
Drivers
- Increasing prevalence of dry eye disease, especially among aging populations.
- Growing demand for long-acting therapies that enhance adherence.
- Improvements in delivery technology and procedural safety.
Barriers
- Higher upfront cost and procedural complexity.
- Competition from established eye drop therapies with strong brand loyalty.
- Limited provider familiarity with implant procedures initially.
Sales Projections
Assumptions
- Market penetration starts at a modest level, growing as clinician familiarity and patient acceptance increase.
- CAGR driven by increased prevalence, improved patient compliance, and expanding geographic access.
- Competitive dynamics remain relatively stable, though novel biologics and generic entries could influence market share.
Projection Overview (2023-2027)
| Year | Estimated Total Market (USD millions) | Restasis OP Market Share | Estimated Sales (USD millions) |
|---|---|---|---|
| 2023 | 4,500 | 2% | 90 |
| 2024 | 4,785 | 3% | 143 |
| 2025 | 5,089 | 4% | 204 |
| 2026 | 5,417 | 5% | 271 |
| 2027 | 5,769 | 6% | 346 |
Notes:
- The increasing market share reflects growing clinician acceptance, expanded indications, and geographic expansion.
- The CAGR for sales over this period approximates 50%, considering initial low adoption and aggressive market penetration efforts.
- These projections assume continued technological advantages and favorable reimbursement policies.
Long-term Outlook (2028-2030)
- As awareness and procedural adoption grow, Restasis OP could secure a 7-10% market share, translating into USD 400-500 million in global sales.
- The potential expansion into other ophthalmic inflammatory indications could further augment revenues.
Strategic Insights
- Market Penetration: Focus on educating ophthalmologists about long-acting benefits and procedural safety to accelerate adoption.
- Pricing Strategy: Balance between recouping R&D investments and competitive positioning against drop-based therapies.
- Geographic Expansion: Prioritize markets with high dry eye prevalence, aging demographics, and receptive healthcare systems.
- Pipeline Development: Invest in complementary formulations or delivery devices to sustain growth and fend off competition.
Key Takeaways
- Restasis OP emerges as a significant player in the evolving dry eye treatment landscape, driven by its sustained-release technology that addresses compliance barriers.
- Market projections indicate rapid growth potential, with sales expected to reach USD 346 million by 2027, contingent on market acceptance and reimbursement conditions.
- Competitive advantage hinges on clinician education, procedural safety, and demonstrating superior adherence benefits.
- Strategic expansion into global markets and indications will be crucial for maximizing revenue streams.
- The therapy’s success will largely depend on balancing market penetration strategies with managing procedural costs and reimbursement frameworks.
FAQs
1. What advantages does Restasis OP offer over traditional cyclosporine eye drops?
Restasis OP provides sustained drug release over several months, reducing the frequency of administration and improving patient adherence, which is a common challenge with traditional eye drops.
2. How does the procedural nature of Restasis OP affect its market adoption?
While the implantation procedure introduces procedural costs and training requirements, it also offers long-term treatment benefits. Adoption hinges on provider familiarity, procedural safety, and reimbursement policies.
3. What are the primary barriers to market growth for Restasis OP?
Key barriers include higher upfront costs, procedural complexity, limited clinician awareness, and competition from established topical therapies and emerging biologics.
4. How will reimbursement policies influence the sales trajectory of Restasis OP?
Reimbursement will significantly impact adoption rates. Favorable coverage will facilitate utilization, whereas reimbursement hurdles may limit initial penetration especially in cost-sensitive markets.
5. What are the potential future applications of Restasis OP technology?
Beyond dry eye treatment, the sustained-release platform may be adapted for delivering anti-inflammatory or immunomodulatory agents in other ocular conditions such as uveitis or postoperative inflammation.
Sources
[1] Schaumberg, D. A., et al. "Prevalence of Dry Eye Syndrome among United States Women." American Journal of Ophthalmology, 2003.
[2] MarketsandMarkets. "Dry Eye Disease Market by Diagnosis, Treatment, End User – Global Forecast to 2028."
More… ↓
