Share This Page
Drug Sales Trends for PROMETHAZINE W/ CODEINE
✉ Email this page to a colleague
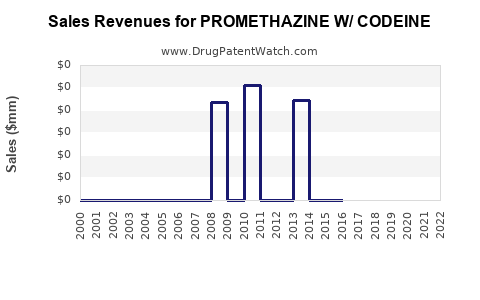
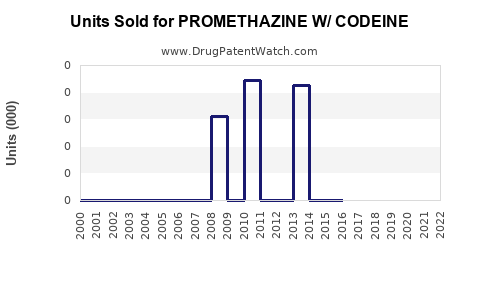
Annual Sales Revenues and Units Sold for PROMETHAZINE W/ CODEINE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| PROMETHAZINE W/ CODEINE | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for Promethazine with Codeine
Overview of Promethazine with Codeine
Promethazine combined with codeine, a prescribed opioid-antihistamine formulation, has historically served as an effective treatment for cough suppression, allergy relief, and nausea management. Marketed under various brand names—such as Phenergan with Codeine—the drug has been integral in symptomatic relief for respiratory illnesses, pain, and allergy concerns. However, increasing regulatory scrutiny, concerns regarding opioid abuse, and the shift toward alternative therapies are reshaping its market landscape.
Regulatory Environment and Market Constraints
Regulations globally have tightened around opioids, especially regarding formulations like promethazine with codeine. The U.S. Drug Enforcement Agency (DEA) classifies codeine as a Schedule III substance, limiting prescribing practices and refilling policies [1]. Several jurisdictions have imposed restrictions or outright bans on over-the-counter (OTC) sales of codeine-containing medications, impacting accessibility and, subsequently, market size.
Furthermore, the rising opioid epidemic in various countries prompts stricter controls, increased monitoring, and potential legislation aimed at curbing misuse. For example, European nations have reclassified codeine as a prescription-only medicine, reducing non-medical use but affecting sales volumes [2].
Market Dynamics and Key Drivers
Demand Factors
- Therapeutic Need: Despite concerns, the drug remains an analgesic and antitussive agent for specific patient populations. Its efficacy in managing cough and allergy symptoms continues to sustain demand.
- Prescribing Trends: Healthcare providers increasingly favor non-opioid alternatives to mitigate abuse potential. The shift influences prescriber habits, especially as guidelines recommend safer, over-the-counter or non-opioid options.
- Patient Awareness: Growing public awareness regarding opioid dependence deters many from using promethazine with codeine, affecting demand.
Supply Factors
- Manufacturing Constraints: Stricter manufacturing standards and licensing requirements can limit supply.
- Market Entry Barriers: Patent expirations and generic proliferation lower prices but do not necessarily expand market size.
Competitive Landscape
- Alternatives: Non-opioid cough suppressants and antihistamines, such as dextromethorphan or loratadine, are increasingly substituting promethazine with codeine.
- Emerging Therapies: New formulations with improved safety profiles or targeted delivery systems are poised to replace traditional products.
Geographic Market Analysis
United States
The U.S. market faced significant decline after regulatory actions; sales have plummeted due to the DEA's rescheduling of codeine products and the opioid epidemic. The CDC and FDA issued warnings highlighting abuse potential, leading to withdrawal from many pharmacies and a reduction in prescribing [3].
Europe
Europe exhibits a more restrictive environment; many countries have shifted promethazine with codeine to prescription-only or banned OTC sales. While this constrains short-term sales, it may promote safer usage and eventually stabilize demand among licensed prescribers.
Asia-Pacific
Emerging markets in Asia-Pacific still exhibit substantial demand, driven by over-the-counter availability, but increasingly face regulatory scrutiny, which could influence future sales.
Sales Projections (2023-2028)
Assumptions
- Market Decline: The U.S. and Europe will see continued decline in sales owing to regulatory restrictions and the opioid crisis.
- Emerging Markets: Slight growth as regulatory landscapes loosen or as markets remain permissive.
- Synthetic Alternatives: Growth of non-opioid alternatives reduces traditional drug demand.
Forecast Overview
| Year | Global Sales (USD Millions) | CAGR | Key Factors |
|---|---|---|---|
| 2023 | $220 | — | Post-pandemic stabilization, ongoing restrictions |
| 2024 | $190 | -13.6% | Increased regulatory enforcement |
| 2025 | $160 | -15.8% | Continued decline in Western markets |
| 2026 | $135 | -15.6% | Market saturation, substitution trends |
| 2027 | $115 | -14.8% | Emergence of safer alternatives |
| 2028 | $100 | -13.0% | Market contraction stabilizes at lower levels |
Note: The overall downtrend reflects regulatory impacts and shifting medical practices. Growth in certain regions may temporarily buffer declines but are unlikely to reverse the long-term trend.
Implications for Stakeholders
- Manufacturers: Diversify portfolios toward non-opioid formulations and develop safer alternatives.
- Investors: Exercise caution in research and development projects relying heavily on promethazine with codeine.
- Healthcare Providers: Focus on prescribing guidelines aligned with current safety standards.
- Regulators: Balance access with abuse deterrence; monitor emerging misuse trends.
Key Takeaways
- The global market for promethazine with codeine is undergoing a significant decline driven primarily by regulatory restrictions and opioid misuse concerns.
- Western markets, particularly the U.S. and Europe, exhibit strong downward trends, which are unlikely to reverse without paradigm-shifting innovations.
- Emerging markets may present limited opportunities, but increased regulation could temper growth.
- Future success hinges on reformulating or replacing promethazine with codeine with safer, non-opioid alternatives.
FAQs
1. What are the primary factors affecting sales of promethazine with codeine?
Regulatory restrictions, opioid misuse concerns, prescriber preferences shifting toward non-opioid therapies, and increased availability of alternatives significantly impact sales.
2. Are there legal restrictions on prescribing promethazine with codeine?
Yes. In many jurisdictions, especially in the U.S. and Europe, regulations classify promethazine with codeine as a controlled substance, constraining prescribing practices and access.
3. What are potential alternatives to promethazine with codeine?
Non-opioid cough suppressants such as dextromethorphan, antihistamines like loratadine, and newer formulations with improved safety profiles serve as substitutes.
4. How might emerging therapies influence future sales?
Development of non-addictive, targeted therapies could further displace promethazine with codeine, leading to sustained declines in traditional formulations.
5. Is there any residual market for promethazine with codeine?
Some niche markets, especially in regions with lax regulations, continue to utilize promethazine with codeine. However, overall demand is diminishing.
References
[1] DEA Scheduling Information, U.S. Drug Enforcement Administration, 2022.
[2] European Medicines Agency, Reclassification of Codeine-Containing Medicines, 2021.
[3] CDC Guidelines on Opioid Prescribing and Abuse, 2020.
More… ↓
