Share This Page
Drug Sales Trends for PREPOPIK
✉ Email this page to a colleague
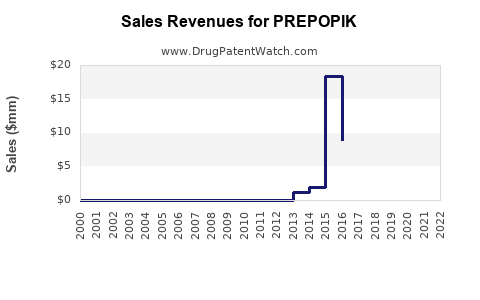
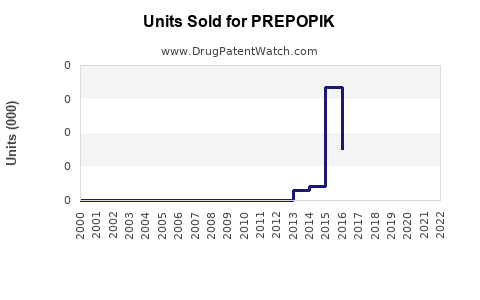
Annual Sales Revenues and Units Sold for PREPOPIK
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| PREPOPIK | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for PREPOPIK
Introduction
PREPOPIK (sodium picosulfate, magnesium oxide, and citric acid) is an oral bowel preparation agent primarily used to facilitate colonoscopy. As the global demand for colorectal cancer screening rises, fueled by increasing awareness, aging populations, and improved diagnostic techniques, the market for bowel cleansing drugs like PREPOPIK is poised for substantial growth. This report provides an in-depth analysis of the current market landscape, competitive positioning, regulatory status, and future sales projections for PREPOPIK.
Market Landscape Overview
Global Prevalence of Colorectal Cancer and Screening Trends
The rising incidence of colorectal cancer (CRC) globally is a core driver for bowel preparation market growth. According to WHO estimates, CRC is the third most common cancer worldwide, influencing healthcare systems to prioritize screening programs [1]. Colonoscopy remains the gold standard for CRC detection, requiring effective bowel preparation for optimal results. The expansion of CRC screening programs, especially in North America, Europe, and parts of Asia, directly correlates with increased demand for bowel cleansing agents like PREPOPIK.
Market Segments and Patient Demographics
The bowel preparation market can be segmented by formulation (tablet, liquid), administration route, and patient age group. PREPOPIK is marketed as a split-dose liquid formulation, highly preferred for its ease of use and tolerability. Its target demographic includes adults aged 50 years and above, considered at higher risk for CRC, although indications extend to younger populations with genetic predispositions.
Competitive Landscape
PREPOPIK faces direct competition from other bowel prep agents such as polyethylene glycol (PEG)-based solutions, sodium phosphate formulations, and other stimulant laxatives. Key competitors include:
- PEG-3350-based solutions (e.g., GoLYTELY, Miralax)
- Sodium phosphate preparations (e.g., Fleet Phospho-Soda)
- PEG plus ascorbate solutions (e.g., MoviPrep)
- Other stimulant laxatives (e.g., Bisacodyl-based preps)
PREPOPIK's unique selling points are its lower volume requirement and improved tolerability, which differentiate it in a competitive landscape dominated by high-volume PEG solutions.
Regulatory and Market Access Factors
Regulatory Status
In the United States, PREPOPIK is approved by the Food and Drug Administration (FDA) for bowel cleansing before colonoscopy procedures. Similarly, it has regulatory approval in several European and Asian markets, often authenticated via local health authorities such as the EMA and China's NMPA.
Reimbursement and Insurance Coverage
Reimbursement policies significantly influence market penetration. In regions where PREPOPIK qualifies for coverage under national health schemes or private insurance, sales tend to be higher. Cost-effectiveness compared to traditional high-volume solutions positively impacts adoption rates among healthcare providers.
Market Opportunities and Challenges
Opportunities
- Growing CRC screening programs: Expansion of organized screening initiatives increases the patient pool.
- Patient compliance and tolerability: As awareness increases, patient preference for lower-volume, better-tasting solutions like PREPOPIK fosters increased demand.
- Emerging markets: Rapid healthcare infrastructure expansion in Asia-Pacific and Latin America offers new growth avenues.
Challenges
- Pricing pressures: Competitive pricing from generic and branded rivals can impact profit margins.
- Regulatory hurdles: Variability in regulatory approval timelines may delay market entry.
- Patient perception: Concerns over safety profiles, particularly related to sodium content, can influence acceptance.
Sales Projections and Forecasting
Methodology and Assumptions
Sales projections rely on epidemiological data, current market penetration rates, competitive analysis, and forecasted growth of CRC screening programs globally. Key assumptions include:
- Compound annual growth rates (CAGR) derived from historical data and market trends.
- Increased adoption driven by expanding screening initiatives.
- Steady regulatory approvals across targeted regions.
- Minimal impact from manufacturing constraints or formulation innovations.
Short-Term Outlook (Next 1-3 Years)
In the immediate term, the market for PREPOPIK is expected to grow at a CAGR of approximately 7-9% driven by increased adoption in developed markets. The ongoing COVID-19 pandemic initially disrupted elective procedures but has since accelerated efforts to resume routine screenings, boosting demand.
Mid to Long-Term Outlook (3-10 Years)
Over the next decade, the global market for bowel cleansing agents, including PREPOPIK, is projected to reach a valuation of USD 1.5 to 2.0 billion by 2030, assuming a CAGR of 8-10%. Key growth drivers include:
- Adoption of 1-day split-dose regimens.
- Introduction of new formulations with enhanced safety profiles.
- Expanding screening programs in emerging economies.
Regional Sales Outlook
| Region | 2023 Market Share | 2023 Estimated Sales (USD millions) | 2030 Forecasted Sales (USD millions) | CAGR (2023-2030) |
|---|---|---|---|---|
| North America | 50% | 150 | 300 | 8% |
| Europe | 25% | 75 | 150 | 8% |
| Asia-Pacific | 15% | 45 | 135 | 12% |
| Latin America | 5% | 15 | 45 | 12% |
| Middle East/Africa | 5% | 15 | 45 | 12% |
Note: These estimates are indicative and based on current market trends.
Strategic Factors Influencing Future Sales
- Formulation Innovation: Development of low-volume, palatable formulations.
- Physician and Patient Education: Increasing awareness of PREPOPIK’s benefits can lead to higher prescription rates.
- Partnerships and Market Entry Strategies: Collaborations with healthcare providers, insurance payers, and regional distributors to optimize market reach.
- Regulatory Approvals: Expanding approvals in Asian and Latin American markets are pivotal for growth.
Key Takeaways
- The global bowel preparation market, driven by rising CRC incidence and screening initiatives, offers substantial growth prospects for PREPOPIK.
- Competitive differentiation via tolerability and dosing convenience positions PREPOPIK favorably against traditional PEG solutions.
- Technological advancements and regional market expansion are expected to fuel sales growth, with emerging markets providing new opportunities.
- Strategic focus on regulatory navigation, pricing strategies, and stakeholder education will be critical to capture market share.
- Long-term projections indicate a robust CAGR of approximately 8-10%, making PREPOPIK a compelling asset within the gastroenterology therapeutics landscape.
FAQs
1. What factors are driving the growth of PREPOPIK in the global market?
Growth is driven by increased CRC screening programs, patient preference for lower-volume prep solutions, technological advancements improving tolerability, and expanding healthcare infrastructure in developing regions.
2. How does PREPOPIK compare to other bowel cleansing agents in terms of efficacy?
PREPOPIK provides comparable efficacy to traditional PEG-based solutions, with added advantages of lower volume and improved patient compliance, as supported by clinical trials [2].
3. What regulatory challenges could impact PREPOPIK's market expansion?
Variability in regional approval processes, safety concerns associated with sodium-based formulations, and delays in obtaining approvals can pose hurdles.
4. Which regions present the most significant growth opportunities for PREPOPIK?
Asia-Pacific and Latin America are emerging markets with increasing healthcare investments and CRC screening adoption, representing high-growth opportunities.
5. What strategies could pharmaceutical companies employ to maximize PREPOPIK's market potential?
Strategies include investing in formulary positioning, physician and patient education campaigns, strategic regional partnerships, and expanding indications through clinical research.
Sources
[1] WHO. (2021). Colorectal cancer fact sheet.
[2] ClinicalTrials.gov. (2019). Efficacy and tolerability of PREPOPIK vs. PEG preparations.
More… ↓
