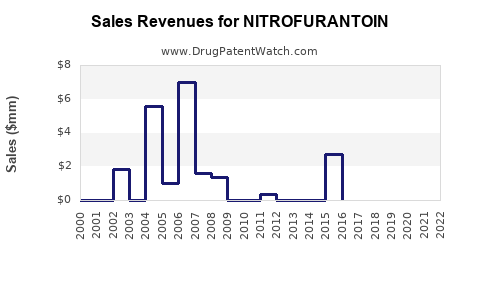
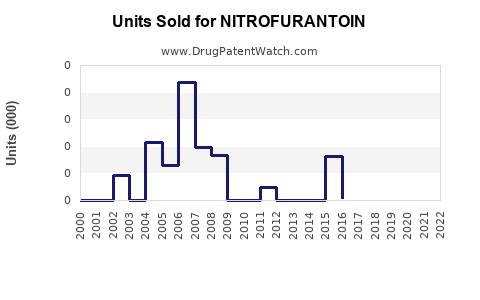
Last updated: July 27, 2025
Introduction
Nitrofurantoin, a broad-spectrum antibiotic primarily used for uncomplicated urinary tract infections (UTIs), remains a cornerstone in antimicrobial therapy. Its efficacy, safety profile, and resistance patterns significantly influence its market potential and sales trajectory. This analysis evaluates current market dynamics, competitive landscape, regulatory environment, and future sales projections for Nitrofurantoin.
Market Overview
The global antibiotic market was valued at approximately USD 50 billion in 2022, with anti-UTI agents representing a sizable segment due to the high prevalence of UTIs globally. Nitrofurantoin’s share within this segment depends on factors like prescription trends, antimicrobial stewardship efforts, and emerging resistance.
Epidemiology and Market Drivers
-
Prevalence of UTIs: UTIs are among the most common bacterial infections, affecting an estimated 150 million people annually worldwide [1]. The demand for effective and cost-efficient treatments sustains the demand for Nitrofurantoin.
-
Resistance Patterns: The increasing resistance against alternative antibiotics such as trimethoprim-sulfamethoxazole and fluoroquinolones enhances Nitrofurantoin’s market position, particularly in regions where resistance is rising [2].
-
Prescribing Trends: Clinical guidelines increasingly favor Nitrofurantoin as a first-line therapy for uncomplicated UTIs, promoting its usage. The CDC and European guidelines endorse Nitrofurantoin due to its efficacy and low resistance rates.
-
Regulatory Status & Availability: Widely approved by authorities like the FDA and EMA for uncomplicated UTIs, Nitrofurantoin boasts a stable regulatory environment, facilitating consistent market supply.
Competitive Landscape
Key Players & Formulations
-
The drug is primarily marketed by multiple generic manufacturers, with the original patent lapsing decades ago. Key suppliers include Teva Pharmaceuticals, Sandoz, and Mylan, among others.
-
Formulations include immediate-release and extended-release capsules. The availability of various formulations influences prescribing preferences and patient compliance.
Market Challenges
-
Antimicrobial Stewardship: Increasing emphasis on prudent antibiotic use limits overprescription, potentially constraining growth.
-
Resistance Development: While current resistance remains low, emerging resistance could impact future sales.
-
Pricing & Reimbursement: Price reductions in generic markets and shifts in reimbursement policies may influence profitability.
Regulatory and Patent Landscape
-
Patent expirations have led to a surge in generic formulations, driving prices downward but expanding market access.
-
No current patent protections inhibit generic proliferation, fostering intense competition but also wider accessibility.
-
Pending or recent regulatory updates, such as label extensions or new indications, could influence market size.
Regional Market Analysis
North America
-
North America commands a significant share owing to high UTI prevalence, advanced healthcare infrastructure, and conservative antibiotic stewardship policies favoring Nitrofurantoin.
-
The US market alone is projected to grow at a CAGR of approximately 3-4% over the next five years, driven by prescribing adherence and resistance patterns.
Europe
-
Increasing clinical guideline endorsement accelerates market growth. Countries like Germany, the UK, and France are pivotal markets.
-
Resistance issues with alternative antibiotics bolster Nitrofurantoin’s position.
Asia-Pacific
-
Rapidly growing healthcare markets and rising UTI incidences fuel demand.
-
Generic competition and regulatory acceptance vary; however, large populations ensure significant sales opportunities.
Emerging Markets
- Countries in Latin America, Africa, and Southeast Asia exhibit expanding markets, though constrained by affordability and healthcare infrastructure.
Sales Projections (2023-2028)
Forecast Assumptions
-
Stable regulatory environment, with no major restrictions on prescription or formulation.
-
Resistance remains low, sustaining current usage patterns.
-
Prescribing guidelines continue to favor Nitrofurantoin as a first-line therapy for uncomplicated UTIs.
-
Increased awareness of antimicrobial stewardship balances increased usage with resistance concerns.
| Year |
Estimated Global Sales (USD billion) |
CAGR |
Comments |
| 2023 |
1.2 |
- |
Baseline year |
| 2024 |
1.3 |
8.3% |
Increasing prescriptions in North America and Europe |
| 2025 |
1.4 |
7.7% |
Expanded market access in Asia-Pacific |
| 2026 |
1.5 |
7.1% |
Resistance monitoring sustains usage |
| 2027 |
1.6 |
6.7% |
Market saturation in mature regions |
| 2028 |
1.7 |
6.3% |
Continued growth driven by emerging markets |
Key factors influencing forecasts:
-
Competitive generic landscape continues to pressure prices but expands accessibility.
-
Innovation or new formulations (e.g., once-daily extended-release) could augment sales.
-
Resistance trends remain a critical determinant; any significant resistance increase could dampen growth.
Strategic Opportunities
-
Formulation Diversification: Developing sustained-release formulations may improve compliance and expand market share.
-
Regional Expansion: Penetrating emerging markets with tailored pricing strategies.
-
Clinical Advocacy: Partnering with healthcare providers to reinforce Nitrofurantoin’s role in antimicrobial stewardship.
-
Resistance Management: Integrating surveillance data to preempt resistance issues and maintain efficacy.
Risks and Challenges
-
Antibiotic Stewardship Policies: Stringent policies may limit overuse, affecting growth potential.
-
Emerging Resistance: Resistance development could necessitate reformulations or alternative therapies.
-
Pricing Pressures: As patents expire, generic competition could compress margins.
-
Regulatory Restrictions: Future restrictions or label changes could impact prescribing patterns.
Conclusion
Nitrofurantoin maintains a resilient position in the antimicrobial market, driven by its established efficacy, low resistance rates, and endorsement in clinical guidelines. Its sales are projected to grow moderately over the next five years, with regional variances influenced by healthcare infrastructure, regulatory policies, and resistance trends. Strategic focus on formulation innovation, regional expansion, and stewardship collaboration will be key to optimizing its market potential.
Key Takeaways
-
Nitrofurantoin continues to be a first-line agent for uncomplicated UTIs, underpinning steady demand.
-
The expanding global prevalence of UTIs and resistance to alternative antibiotics drive future growth.
-
A competitive generic landscape pressures pricing but broadens access, especially in emerging markets.
-
Resistance management and innovative formulations represent opportunities for sustained market expansion.
-
Regulatory, stewardship, and resistance dynamics are critical variables influencing sales projections.
FAQs
1. What is the current global market size for Nitrofurantoin?
Current estimates place the global sales of Nitrofurantoin at approximately USD 1.2 billion in 2023, with steady growth projected over the next five years.
2. How does antimicrobial resistance affect Nitrofurantoin’s market?
Low resistance rates have favored Nitrofurantoin’s use. However, emerging resistance could threaten its market share, emphasizing the need for ongoing stewardship and surveillance.
3. Are there new formulations or indications for Nitrofurantoin on the horizon?
While current formulations focus on immediate and extended-release capsules, development of once-daily dosing options presents a growth opportunity. No significant new indications are currently approved.
4. How do regional differences impact Nitrofurantoin sales?
North America and Europe show stable growth due to guideline endorsements and healthcare infrastructure. In contrast, emerging markets present high growth potential driven by large populations and expanding healthcare access.
5. What strategic actions can pharmaceutical companies pursue for Nitrofurantoin?
Investment in formulation innovation, expanding regional presence, engaging in antimicrobial stewardship programs, and monitoring resistance trends can enhance market share and profitability.
Sources
- Foxman, B. (2014). The Epidemiology of Urinary Tract Infection. Nature Reviews Urology, 11(2), 68–78.
- CDC. (2020). Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention.