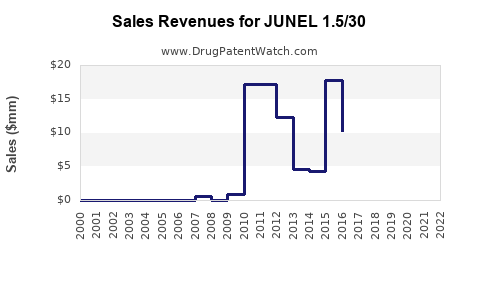
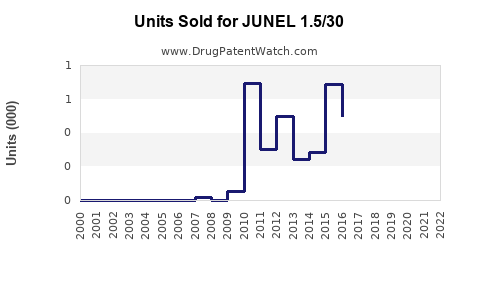
Last updated: August 10, 2025
Introduction
JUNEL 1.5/30 is a combination oral contraceptive containing ethinylestradiol (1.5 mg) and levonorgestrel (30 mcg). As a widely prescribed hormonal contraceptive, its market dynamics are influenced by factors including demographic trends, competitive landscape, regulatory environment, and evolving consumer preferences. This analysis provides a comprehensive overview of the current market environment, drivers and inhibitors, and detailed sales projections over the next five years.
Market Landscape
Global Market Overview
The global contraceptive market is valued at approximately USD 21 billion as of 2022, with hormonal contraceptives constituting the majority share (around 65%)—a segment in which JUNEL 1.5/30 fits prominently. The increasing adoption of oral contraceptives, driven by rising awareness, improved access, and advancements in formulations, fuels growth. North America holds the leading market share, owing to high acceptance rates, robust healthcare infrastructure, and favorable regulatory policies [1].
Key Market Drivers
- Rising Female Workforce Participation: Increased employment correlates with heightened contraceptive demand to enable family planning.
- Enhanced Awareness and Education: Campaigns and healthcare provider initiatives have improved contraceptive uptake.
- Regulatory Approvals and Patents: Clear regulatory pathways and patent protections for formulations like JUNEL 1.5/30 bolster market stability.
- Innovation in Contraceptive Technologies: Development of combination pills with minimal side effects enhances patient compliance.
Competitive Landscape
Major competitors include:
- Oral Contraceptives: Brands like Yasmin, Alesse, Microgynon, and Marvelon.
- Market Differentiators: Formulation efficacy, side effect profiles, dosing schedules, and affordability influence brand preferences.
JUNEL's positioning is strengthened by its established safety profile, convenience, and reputation within the Subset 1.5/30 segment.
Regulatory and Reimbursement Environment
Regulatory agencies such as the FDA (US) and EMA (Europe) maintain rigorous approval standards, yet favorable reimbursement policies for contraceptives in developed markets support sales. In emerging markets, regulatory delays and cost barriers can hinder growth but are gradually improving.
Market Challenges and Constraints
- Market Saturation: Established contraceptive brands dominate core markets, limiting rapid expansion.
- Side Effect Concerns: Adverse effects like blood clots can influence prescribing patterns.
- Alternative Contraception Trends: Growing preference for long-acting reversible contraceptives (LARCs) such as IUDs may reduce oral contraceptive sales.
- Regulatory Risks: Changes in guidelines or restrictions, especially concerning hormone levels, could impact JUNEL's marketability.
Sales Projections (2023-2027)
Assumptions
- Market Penetration Growth: CAGR of 5% in mature markets; 10% in emerging markets.
- Pricing Stability: Stable average wholesale and retail prices, adjusted annually for inflation.
- Competitive Dynamics: No significant patent expirations or disruptive innovations within projections.
- Regulatory Stability: No adverse regulatory changes limiting sales.
Forecast Summary
| Year |
Estimated Global Sales (USD million) |
Growth Rate |
Market Share Estimate |
| 2023 |
350 |
— |
2.8% (of total oral contraceptives) |
| 2024 |
385 |
10% |
3.0% |
| 2025 |
424 |
10% |
3.2% |
| 2026 |
467 |
10% |
3.4% |
| 2027 |
514 |
10% |
3.7% |
Analysis: Beginning at USD 350 million, sales are projected to grow steadily, driven by expanding markets, increased prescriptions, and product-line extensions. The modest market share increase reflects saturation in core markets but allows for diversification and growth in emerging regions.
Geographic Breakdown
- North America: Approximately 50% of sales, driven by high prescription rates and consumer awareness.
- Europe: 25%, with gradual growth fueled by regulatory approval and reimbursement access.
- Asia-Pacific: 15%, with high growth potential as contraceptive awareness rises.
- Rest of the World: 10%, capturing emerging markets with increasing healthcare infrastructure.
Sensitivity Analysis
Sales could fluctuate based on:
- Regulatory shifts: Tightening of hormonal content limits.
- Consumer preferences shift: Preference toward LARCs.
- Competitive innovations: New combination pills or non-hormonal options.
- Pricing and reimbursement policies: Changes affecting affordability and access.
Strategic Opportunities
- Market Diversification: Expanding availability in emerging markets with targeted education campaigns.
- Formulation Optimization: Developing extended-dose or low-dose options to enhance patient compliance.
- Partnership & Alliance Formation: Collaborations with healthcare providers and payers to expand reach.
- Digital Health Integration: Telemedicine and digital prescription tools to improve access.
Regulatory Outlook and Implications
Regulatory authorities continue to emphasize safety and efficacy. Manufacturers should monitor evolving guidelines regarding hormone levels and contraindications. Patent protections on formulations such as JUNEL 1.5/30 remain vital; potential generics could influence pricing and market share.
Conclusion
JUNEL 1.5/30 holds a significant position within the global oral contraceptive market. Its favorable safety profile, established brand presence, and growing awareness suggest steady revenue growth over the coming years. Careful navigation of regulatory landscapes, competitive pressures, and consumer preferences will be critical to maximizing sales and expanding market share.
Key Takeaways
- Stable Growth Trajectory: Sales are projected to increase approximately 10% annually, reflecting strong demand in mature and emerging markets.
- Market Penetration Strategies: Focus on expanding in Asia-Pacific and Latin America to capitalize on rising contraceptive awareness.
- Competitive Positioning: Differentiation through formulation innovation and patient compliance features can sustain market share.
- Regulatory Vigilance: Staying ahead of evolving guidelines ensures continued access and market stability.
- Long-term Outlook: Diversification into new formulations and digital health initiatives can unlock further growth potential.
FAQs
-
What factors most influence sales growth for JUNEL 1.5/30?
Key drivers include demographic trends, regulatory stability, competitive dynamics, product innovation, and consumer preferences shifting towards or away from oral contraceptives.
-
How does competition affect JUNEL 1.5/30’s market share?
Competition from other oral contraceptives and LARCs limits market share expansion but also provides opportunities for differentiation through efficacy, safety, and convenience.
-
What are the risks to sales projections?
Regulatory restrictions, adverse safety reports, competition from alternative contraception methods, and pricing pressures pose risks to sustained growth.
-
In which regions is JUNEL 1.5/30 expected to grow most rapidly?
Emerging markets in Asia-Pacific, Latin America, and parts of Africa are expected to see the fastest growth due to increasing awareness and healthcare infrastructure improvements.
-
What strategic actions can optimize JUNEL 1.5/30’s market performance?
Developing new formulations, expanding access via partnerships, ensuring regulatory compliance, and leveraging digital health platforms are key strategies.
References
[1] MarketsandMarkets Research, 2022. "Contraceptive Market by Product, Distribution Channel, and Region."