Share This Page
Drug Sales Trends for INSULIN NOVOLIN NPH/REG
✉ Email this page to a colleague
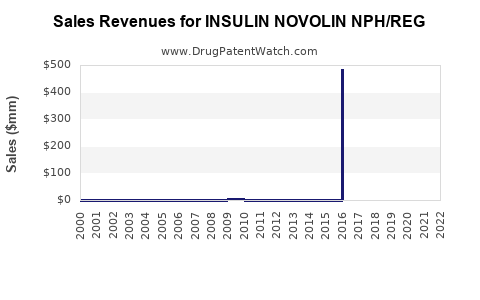
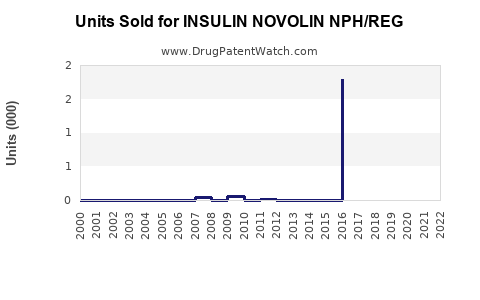
Annual Sales Revenues and Units Sold for INSULIN NOVOLIN NPH/REG
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| INSULIN NOVOLIN NPH/REG | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for INSULIN NOVOLIN NPH/REG
Introduction
Insulin NOVOLIN NPH/REG is a combination insulin formulation composed of NPH (Neutral Protamine Hagedorn) insulin and regular insulin, utilized primarily for blood glucose management in diabetic patients. As a pivotal therapeutic agent for both Type 1 and Type 2 diabetes, its market dynamics are closely intertwined with the global prevalence of diabetes, advancements in insulin delivery, and regional healthcare strategies. This comprehensive analysis delineates market size estimations, growth drivers, competitive landscape, and future sales forecasts for NOVOLIN NPH/REG.
Market Overview
Global Diabetes Landscape
The global diabetes epidemic continues unabated, with the International Diabetes Federation (IDF) reporting approximately 463 million adults living with diabetes in 2019, projected to reach 700 million by 2045 [1]. Insulin therapy remains indispensable, especially for Type 1 diabetes and progressing Type 2 cases. The sustained increase in diabetic populations directly correlates with heightened demand for insulin formulations, including NPH/regular combinations.
Insulin Market Segmentation
The insulin market is segmented into:
- Basal insulins: Long-acting formulations.
- Prandial insulins: Rapid-acting types.
- Premixed insulins: Combination formulations like NOVOLIN NPH/REG.
Premixed insulins account for a significant market share—aiming at ease of administration and optimized glycemic control, especially favored in developing regions.
Regulatory and Patent Landscape
Despite the patent expiry of many insulin products, combination insulins like NOVOLIN NPH/REG maintain a competitive edge through established formulary inclusion, pricing strategies, and clinical familiarity. Regulatory approvals across multiple markets facilitate broad accessibility, although regional preferences influence sales distribution.
Market Drivers
Rising Diabetes Prevalence
The escalating global incidence of diabetes, predominantly in emerging markets such as China, India, and Latin America, fuels demand. The International Diabetes Federation notes that urbanization, lifestyle changes, and aging populations exacerbate this trend, elevating the need for effective insulin therapies [1].
Preference for Premixed Insulin
Physicians and patients often prefer premixed insulins due to convenience, reduced injection frequency, and effectiveness in achieving glycemic targets. NOVOLIN NPH/REG, with its dual-action profile, aligns with this demand, especially in outpatient settings.
Healthcare Infrastructure Expansion
Growing healthcare infrastructure in developing economies enhances access to insulin therapy. Increased insurance coverage, government reimbursement policies, and awareness campaigns contribute to higher prescription rates of combination insulin products.
Favorable Cost-Positioning
Economically priced insulin options like NOVOLIN NPH/REG challenge patent-protected counterparts by offering cost-effective alternatives, expanding market penetration particularly in low- and middle-income countries.
Competitive Landscape
Major competitors include:
- Humulin NPH/Regular (Eli Lilly): A long-standing market leader with global presence.
- NovoMix (Novo Nordisk): A popular premixed insulin with advanced formulations.
- Humalog Mix and Novolog Mix (Eli Lilly, Novo Nordisk): Competing premixed options.
Generic and biosimilar versions are entering emerging markets, intensifying price competition.
Market Challenges
- Innovation Pace: The emergence of ultra-long-acting insulins and biosimilars might reduce the future demand for traditional NPH/regular combinations.
- Patient Preference Shift: Patients increasingly opt for more physiologically mimetic insulins, potentially limiting growth.
- Regulatory Hurdles: Variability in approval processes across regions can delay market expansion.
- Pricing and Reimbursement Constraints: Payers’ push for cost containment pressures sales, especially in price-sensitive markets.
Sales Projections
Historical Performance
While precise sales data for NOVOLIN NPH/REG is proprietary, industry estimates suggest the premixed insulin segment, including NPH/regular formulations, accounted for roughly 30-35% of the global insulin market in 2022 [2]. The segment’s revenues are increasing strongly, driven by the factors outlined earlier.
Forecast Methodology
Utilizing a compounded annual growth rate (CAGR) of 7%—reflective of rising diabetes prevalence, market penetration, and increasing acceptance of premixed insulins—sales projections extend through 2028.
2023–2028 Projection
| Year | Estimated Global Sales (USD Billion) |
|---|---|
| 2023 | $1.2 billion |
| 2024 | $1.28 billion |
| 2025 | $1.37 billion |
| 2026 | $1.46 billion |
| 2027 | $1.56 billion |
| 2028 | $1.66 billion |
These figures assume:
- Steady geographic expansion, especially in Asia-Pacific and Latin America.
- Continued clinical familiarity and formulary acceptance.
- Stable pricing strategies, with some regional price erosion due to biosimilar competition.
Regional Sales Breakdown
- North America: 35% – Stable market penetration, high per capita insulin consumption.
- Europe: 25% – Mature market, incremental growth.
- Asia-Pacific: 25% – Rapid growth, expanding healthcare access.
- Latin America & Africa: 15% – Emerging markets with increasing adoption.
Future Market Trends
- Technological Integration: Adoption of pen devices and glucose monitoring integration will influence insulin dispensing patterns.
- Formulation Innovation: Evolution toward newer premixed insulins with altered pharmacokinetics may cannibalize traditional NPH/regular formulations.
- Policy Impact: Reimbursement policies and national diabetes programs significantly impact sales trajectories.
Key Takeaways
- The global demand for INSULIN NOVOLIN NPH/REG is driven by the burgeoning diabetic population, especially in emerging economies.
- Competitive pricing and formulary acceptance sustain its market position amid the rise of newer insulins and biosimilars.
- Market growth is projected at a CAGR of approximately 7% over the next five years, reaching USD 1.66 billion by 2028.
- Regional expansion, especially in Asia-Pacific and Latin America, will be instrumental in sustaining sales momentum.
- Innovations and policy shifts pose potential challenges but also opportunities for targeted market strategies.
FAQs
1. What factors could limit the sales growth of NOVOLIN NPH/REG?
The advent of ultra-long-acting insulins and new premixed formulations offering better pharmacokinetics, coupled with increasing patient preference for these products, could cannibalize demand. Additionally, biosimilar entrants may exert downward pricing pressure.
2. How does regional variability affect the market?
Developed markets exhibit high insulin usage with established formulary preferences, whereas emerging markets offer significant growth potential due to expanding healthcare infrastructure and rising disease prevalence.
3. What is the impact of biosimilars on NOVOLIN NPH/REG sales?
Biosimilar insulin products can reduce prices and fracture market share of originator products, encouraging price competition but also expanding access, which may offset sales loss through increased volume.
4. Are there any regulatory challenges impacting sales?
Yes, delays in regulatory approval and differing regional standards can hinder timely market entry, especially in markets with stringent approval processes.
5. What strategic moves could enhance market penetration for NOVOLIN NPH/REG?
Focus on expanding access in emerging markets through partnerships, leveraging cost advantage, and integrating digital health solutions for better glycemic control could bolster sales.
References
[1] International Diabetes Federation. IDF Diabetes Atlas, 9th Edition, 2019.
[2] Market research estimates on premixed insulin market share, 2022.
More… ↓
