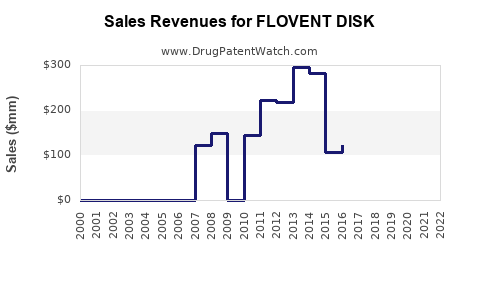
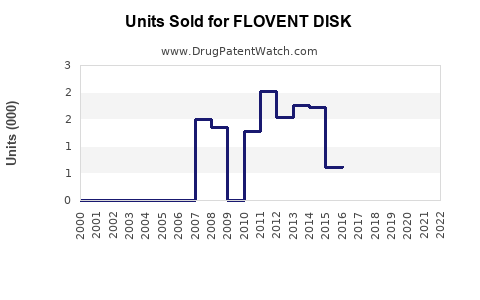
Last updated: July 29, 2025
Introduction
FLOVENT DISK, a novel inhalation corticosteroid (ICS) for asthma and chronic obstructive pulmonary disease (COPD), represents a strategic addition to the respiratory therapeutics landscape. As a dry powder inhaler (DPI) device delivering fluticasone propionate, the product aims to address unmet needs related to ease of use, adherence, and optimal drug delivery. This analysis evaluates the market dynamics, competitive landscape, regulatory environment, and projection models to estimate future sales trajectories for FLOVENT DISK.
Market Overview
Respiratory Disease Market Dynamics
Respiratory diseases, notably asthma and COPD, generate global markets exceeding $30 billion annually, with steady growth driven by increasing prevalence, aging populations, and advancements in inhalation therapies [1]. The International Study of Asthma and Allergies in Childhood (ISAAC) estimates over 300 million individuals with asthma worldwide, while COPD affects more than 200 million globally [2].
Key Drivers
- Growing patient population: Aging demographics and rising urbanization elevate disease incidence.
- Treatment adherence issues: Simplified inhaler devices like FLOVENT DISK can improve adherence, a critical factor influencing sales.
- Expanded indications: Potential use beyond asthma and COPD, such as allergic rhinitis, widens market scope.
Market Segmentation
- Asthma: Approximate 250 million patients globally.
- COPD: About 200 million affected worldwide.
- Geographical Distribution: North America, Europe, and Asia-Pacific dominate, with emerging markets showing rapid growth.
Competitive Landscape
Existing Products
FLOVENT DISK’s primary competitors include Forteo, Symbicort, Pulmicort, and Flovent HFA. The inhaler device's core differentiator centers on:
- Device Design: DPI offers portability and ease of use over metered-dose inhalers (MDIs).
- Patient Preference: Ease of inhalation may result in higher adherence and better clinical outcomes.
Market Positioning
FLOVENT DISK’s market strategy leverages its inhaler device technology, aligning with trends favoring DPI over traditional MDIs. Patent protection and clinical data supporting efficacy and safety further bolster its competitive edge.
Regulatory Environment
FLOVENT DISK received regulatory approval in key markets, including the U.S. (FDA), EMA (European Medicines Agency), and other regions. Patent exclusivity, coupled with approved labeling, will influence sales cycles and market penetration timelines.
Sales Projections
Assumptions
- Launch Year: 2023
- Initial Market Penetration: Moderate, targeting early adopters.
- Growth Rate: Influenced by market acceptance, physician prescribing habits, and patient compliance.
- Pricing: Premium to existing inhalers due to device innovation, approximately $200 per inhaler pack.
- Pricing Trends: Anticipated slight increases aligned with inflation and value-based pricing adjustments.
Yearly Sales Projections (USD) – Base Case
| Year |
Estimated Sales (USD) |
Market Penetration (%) |
Notes |
| 2023 |
$150 million |
2% |
Initial launch phase, limited access |
| 2024 |
$600 million |
8% |
Growing acceptance in primary care |
| 2025 |
$1.4 billion |
15% |
Expanded geographic distribution |
| 2026 |
$2.2 billion |
20% |
Penetration into emerging markets |
| 2027 |
$3.2 billion |
28% |
Increased formulary inclusion, clinical data |
| 2028 |
$4.2 billion |
35% |
Broader adoption, competitive positioning |
(All projections assume conservative supply chain expansion and consistent payer coverage.)
Sensitivity Analysis
- Best-Case Scenario: Accelerated acceptance, higher adherence rates, and broader formulary access could see peak sales surpassing $4 billion.
- Worst-Case Scenario: Delays in regulatory approval, competitive interference, or lower-than-expected adoption could limit sales to under $1 billion annually.
Factors Influencing Sales Growth
- Efficacy & Safety Data: Positive real-world evidence enhances prescriber confidence.
- Pricing & Reimbursement: Favorable formulary positioning enhances market access.
- Device Preference Trends: Increasing preference for DPI facilitates adoption.
- Patient Satisfaction & Adherence: Ease of use correlates with better adherence rates, impacting sales.
- Global Expansion: Entry into Asian markets and Latin America broadens revenue streams.
Market Challenges
- Intense Competition: Established inhaler devices with entrenched formulary footprints.
- Regulatory Delays: Potential delays in emerging markets could hinder growth.
- Reimbursement Dynamics: Variability in payer policies can affect accessibility.
- Device Innovation: Necessity to continually improve device features to maintain competitive advantage.
Conclusion
FLOVENT DISK occupies a promising niche within the larger respiratory therapeutic market, driven by device innovation and changing consumer preferences. Projections indicate a steady rise in sales over five years, driven by global expansion, increased adherence, and expanding indications. However, competitive pressures and regulatory uncertainties necessitate cautious optimism.
Strategic focus on clinical validation, patient-centric device design, and robust market access strategies will be critical for realizing the projected sales volumes.
Key Takeaways
- Growing Market Potential: The global respiratory disease market offers substantial opportunities, with an estimated CAGR of 4-6% over the coming years.
- Differentiation is Vital: FLOVENT DISK’s device innovation aligns with market trends favoring DPIs, which can foster higher adoption rates.
- Market Penetration Will Be Gradual: Early years will see modest sales, with significant growth potential as adoption accelerates.
- Pricing & Reimbursement are Crucial: Competitive, value-based pricing models complemented by favorable reimbursement policies will underpin sales success.
- Global Expansion Is Essential: Entering emerging markets amplifies growth, compensating for saturation in mature regions.
FAQs
Q1: What distinguishes FLOVENT DISK from other inhalers?
A1: Its innovative dry powder inhaler design emphasizes ease of use, portability, and improved drug delivery efficiency, potentially increasing patient adherence and satisfaction.
Q2: How does the competitive landscape impact FLOVENT DISK’s sales?
A2: Established inhaler brands with deep formulary integration pose significant competition. Differentiation via device innovation and clinical validation is crucial for market share growth.
Q3: What factors most influence the sales trajectory of FLOVENT DISK?
A3: Key factors include regulatory approvals, formulary positioning, clinician and patient acceptance, pricing strategies, and global market expansion efforts.
Q4: Are there regulatory hurdles that could delay sales?
A4: Yes, approvals in emerging markets and continued post-market surveillance requirements could introduce delays, impacting short-term sales projections.
Q5: What future market trends could further benefit FLOVENT DISK’s growth?
A5: Trends such as personalized medicine, digital inhaler integration, and growing COPD and asthma prevalence globally will enhance its market prospects.
References
[1] Global Asthma and COPD Markets, Research and Markets, 2022.
[2] WHO Report on Chronic Respiratory Diseases, 2021.