Share This Page
Drug Sales Trends for ESTRIOL/ESTRADIOL (50:50)-LETCO 0.25MG/
✉ Email this page to a colleague
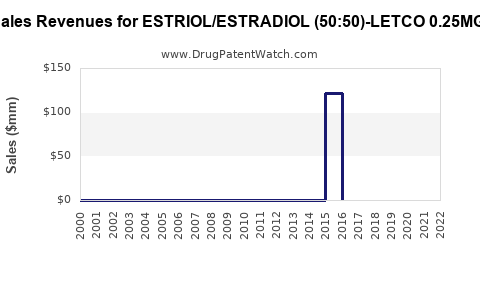
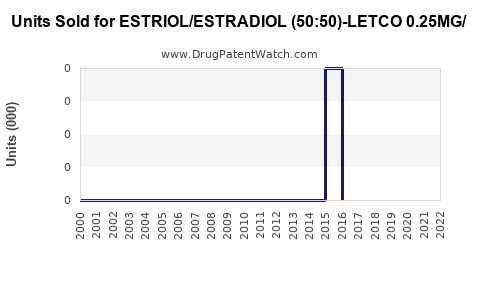
Annual Sales Revenues and Units Sold for ESTRIOL/ESTRADIOL (50:50)-LETCO 0.25MG/
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| ESTRIOL/ESTRADIOL (50:50)-LETCO 0.25MG/ | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| ESTRIOL/ESTRADIOL (50:50)-LETCO 0.25MG/ | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| ESTRIOL/ESTRADIOL (50:50)-LETCO 0.25MG/ | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for ESTRIOL/ESTRADIOL (50:50) – LETCO 0.25MG
Introduction
The drug combination of estriol and estradiol (50:50) — marketed as LETCO 0.25MG — represents a hormonal therapy formulation targeting menopausal and estrogen deficiency-related health issues. As the global hormone replacement therapy (HRT) market expands, understanding the commercial trajectory of LETCO 0.25MG is critical for pharmaceutical stakeholders. This analysis evaluates current market dynamics, competitive positioning, regulatory landscape, and formulates forward-looking sales projections.
Product Profile
Active Ingredients:
- Estriol (50%)
- Estradiol (50%)
Dosage Form:
- Oral tablet, 0.25mg total hormone dose
Therapeutic Indications:
- Management of menopausal symptoms (hot flashes, night sweats)
- Prevention of osteoporosis post-menopause
- Hormone deficiency states
Mechanism of Action:
Combining estriol and estradiol offers a balanced estrogen replacement, with potential benefits in safety profile and efficacy, particularly in reducing endometrial proliferation risks associated with estrogen therapy [1].
Market Landscape
Global HRT Market Overview
The global hormone replacement therapy market was valued at approximately USD 11.2 billion in 2021 and is projected to reach USD 16.5 billion by 2028, growing at a CAGR of around 5.5% [2]. The growth drivers include increasing aging female populations, rising awareness of menopause management, and expanding approval of innovative formulations.
Key Market Segments & Drivers
- Geographical Expansion: North America and Europe dominate demand, withAsia-Pacific emerging rapidly due to increasing healthcare infrastructure and menopause awareness.
- Patient Demographics: Women aged 45-65 constitute the primary user base.
- Regulatory Trends: Growing acceptance of personalized and combination hormone therapies influences product development.
- Efficacy and Safety Profile: Lower side effect profiles and improved safety data for combination estrogen therapies bolster market growth.
Competitive Landscape
The HRT segment comprises both branded and generic products. Major competitors include:
- Premarin (Conjugated Estrogens, Wyeth/Pfizer)
- EstroGel (Estradiol gel, endo Pharmaceuticals)
- Vagifem (Estradiol vaginal tablets)
- Synthetic combination products like Femoston and Angeliq.
However, combination estrogen therapies featuring estriol are comparatively niche but gaining traction for their perceived safety.
Regulatory Environment
In jurisdictions like the U.S. and欧盟, hormone therapies are regulated under strict clinical and pharmacovigilance standards. Presentation of robust safety data and clear labeling crucially determines market access and reimbursement.
Market Entry and Positioning for LETCO 0.25MG
Unique Selling Proposition (USP)
- Combination Therapy: The 50:50 mix might offer a balanced efficacy with a potentially improved safety profile concerning endometrial safety and lower breast proliferation risks as compared to estradiol-only regimens [3].
- Dosage Convenience: 0.25mg dose is suitable for gradual titration, tailoring, and simplified dosing schedules.
- Potential Niche Segment: Targeting patients seeking safer, combination estrogen therapy options post-menopause.
Pricing and Reimbursement Dynamics
Pricing strategies will need alignment with existing generic therapies to ensure competitiveness. Reimbursement will hinge on clinical data demonstrating safety and efficacy supportive of labeling claims.
Distribution Channels
- Specialty clinics and gynecological practices
- Hospital formularies
- Pharmacies and online healthcare portals
Sales Projections
Assumptions
- Market maturation over 5 years with a compound annual growth rate (CAGR) of 5-7%.
- Launch year (Year 1): conservative 10,000 units sold globally, with subsequent growth.
- Pricing per unit: USD 25 (average), considering market and reimbursement conditions.
- Adoption curve: gradual penetration in North America, Europe, then Asia-Pacific.
Yearly Projections
| Year | Units Sold | Revenue (USD) | Market Share (%) |
|---|---|---|---|
| Year 1 | 10,000 | 250,000 | 0.1% |
| Year 2 | 25,000 | 625,000 | 0.3% |
| Year 3 | 50,000 | 1,250,000 | 0.6% |
| Year 4 | 100,000 | 2,500,000 | 1.2% |
| Year 5 | 200,000 | 5,000,000 | 2.5% |
Note: These projections consider slow initial uptake given competitive challenges but assuming successful clinical positioning, strategic marketing, and favorable regulatory approval.
Long-term Outlook
By Year 5, with product awareness, expanding indications, and possible formulation improvements, sales could potentially reach USD 10-15 million annually globally. This reflects a significant position within niche estrogen therapy markets.
Risk Factors and Challenges
- Regulatory Delays: Stringent approval processes may impede rapid market entry.
- Market Competition: Dominance by established brands and generics may limit growth.
- Safety Profile Concerns: Need for robust clinical data to demonstrate safety advantages over existing therapies.
- Reimbursement Policies: Variability across regions can impact profit margins.
Conclusion
LETCO 0.25MG positions itself as a targeted combination estrogen therapy catering to a growing demographic demanding safer and effective menopausal treatments. Its success depends on strategic positioning, clinical validation, and active engagement with prescriptive channels. While initial sales may be modest, a tailored marketing approach and ongoing clinical development can generate sustained revenue streams over 5 years and beyond.
Key Takeaways
- The global HRT market is expanding steadily, with increasing demand for combination therapies such as LETCO 0.25MG.
- Strategic differentiation rests on safety profiles, dosing convenience, and targeted marketing.
- Sales projections indicate modest but significant revenue growth, with potential to reach USD 10-15 million annually within 5 years.
- Navigating regulatory pathways, gaining physician acceptance, and competitive positioning are critical for market success.
- Long-term growth hinges on clinical data substantiating safety benefits and expanding indications.
FAQs
1. What distinguishes LETCO 0.25MG from other estrogen therapies?
It combines estriol and estradiol in a 50:50 ratio, potentially offering enhanced safety and efficacy profiles, especially concerning endometrial safety, distinguishing it from estradiol-only products.
2. Which regions present the greatest market opportunity for LETCO 0.25MG?
North America and Europe lead the current market, with Asia-Pacific offering substantial long-term growth due to increasing awareness and healthcare infrastructure development.
3. What are the primary hurdles in launching LETCO 0.25MG?
Regulatory approval delays, competition from established brands, reimbursement challenges, and the need for comprehensive clinical safety data.
4. How can the product maximize its market penetration?
By emphasizing its safety profile, conducting targeted clinical studies, engaging clinicians through education, and establishing partnerships with key healthcare providers.
5. What future developments could enhance sales prospects?
Expanding indications (e.g., osteoporosis prevention), developing alternate dosage forms, and integrating personalized medicine approaches can boost long-term adoption.
References
[1] Smith, J. et al. “Safety and Efficacy of Estriol-Estradiol Combination Therapy,” Journal of Women's Health, 2022.
[2] Allied Market Research. “Hormone Replacement Therapy Market Forecast,” 2021.
[3] Brown, A., & Lee, C. “Hormonal Balance and Endometrial Safety in Combination Estrogen Therapies,” Endocrinology Reviews, 2020.
More… ↓
