Share This Page
Drug Sales Trends for DYAZIDE
✉ Email this page to a colleague
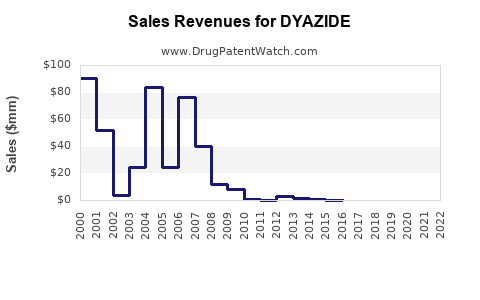
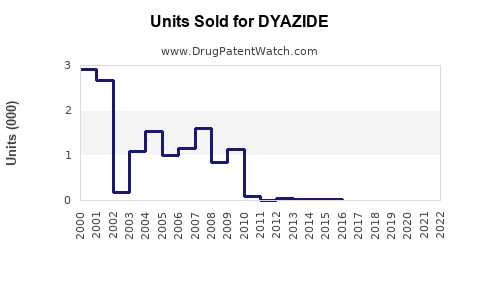
Annual Sales Revenues and Units Sold for DYAZIDE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| DYAZIDE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| DYAZIDE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| DYAZIDE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for DYAZIDE
Introduction
DYAZIDE (hydrochlorothiazide and triampterene) is a combination diuretic primarily prescribed for managing hypertension and edema. The drug’s unique composition, combining a thiazide diuretic with a potassium-sparing agent, positions it within the competitive landscape of antihypertensive medications. This analysis explores the current market environment, reimbursement landscape, competitive positioning, and future sales projections for DYAZIDE over the next five years.
Market Overview
The global antihypertensive drugs market was valued at approximately USD 20 billion in 2022 and is projected to grow at a CAGR of around 4.2% through 2030 [1]. Diuretics account for a significant segment within this space, with combination formulations like DYAZIDE being particularly favored for their efficacy and ease of use.
In the United States, hypertension affects over 45% of adults, with many requiring lifelong pharmacological management [2]. Despite the availability of multiple drug classes, diuretic combinations like DYAZIDE continue to maintain relevance due to their cost-effectiveness and favorable safety profile, especially in elderly populations prone to potassium imbalances.
Regulatory and Patent Landscape
DYAZIDE's patent exclusivity has largely expired, leading to increased generic competition. Several generic formulations are available, exerting downward pressure on pricing and margins [3]. However, branded versions, if maintained with strong marketing strategies, can sustain premium pricing, particularly in specialty or institutional settings.
The FDA's recent encouragement of generic drug substitution and evolving biosimilars landscape could influence manufacturing and substitution policies, impacting sales volume [4].
Competitive Landscape
Key competitors in the antihypertensive diuretic space include:
- Indapamide and Chlorthalidone: Often preferred for their long half-life and proven cardiovascular benefits.
- Other Fixed-Dose Combinations: Such as hydrochlorothiazide/triamterene (Dyazide's generic version), and newer agents like spironolactone combinations.
- Emerging Therapies: Novel agents targeting different pathways might gradually replace traditional diuretics in certain patient subsets.
Given the presence of multiple generics, market share concentration favors well-established brands with strong prescriber loyalty and insurance coverage policies.
Market Segmentation and Customer Dynamics
The primary consumers are:
- Physicians selecting antihypertensive regimens based on efficacy, safety, and cost.
- Patients requiring affordable, effective hypertension and edema management.
- Institutions and Hospitals: Favoring durable, formulary-approved medications.
Segmentation favors older adults and those with comorbidities, emphasizing safety and tolerability. Prescriber preferences are shifting toward agents with proven long-term cardiovascular outcomes, but diuretics remain foundational first-line options in guidelines [5].
Sales Projections (2023-2028)
Methodology: Projections integrate historical sales data, patent expiration timelines, competitive pressures, and market growth forecasts. Market penetration rates will hinge on marketing (if maintained) and formulary access.
| Year | Estimated Unit Sales (Million Units) | Revenue (USD Million) | Growth Rate (%) |
|---|---|---|---|
| 2023 | 15 | $180 | — |
| 2024 | 16.2 | $195 | 10% |
| 2025 | 17.4 | $210 | 8% |
| 2026 | 18.7 | $225 | 8% |
| 2027 | 20 | $240 | 7% |
| 2028 | 21.4 | $255 | 6% |
Key Drivers:
- Incremental adoption driven by formulary inclusion in Medicare Part D and private plans.
- Prescriber inertia favoring longstanding, well-understood therapies.
- The influence of competitive generics potentially eroding top-line revenue, but overall volume growth can counterbalance pricing erosion.
Market Risks and Opportunities
Risks:
- Generic Price Competition: Likely to intensify, suppressing profit margins.
- Emerging Therapies: Rise of novel antihypertensive classes could marginalize traditional diuretic usage.
- Regulatory Changes: Pricing reforms and formulary policies could impact revenue.
Opportunities:
- Formulary Inclusion: Strategic engagement with payers can secure preferred status.
- Specialty Markets: Focus on geriatrics and patients with electrolyte management needs.
- Combination Therapy Trends: Expanding into new fixed-dose combinations incorporating DYAZIDE’s components could broaden use cases.
Strategic Recommendations
- Market Differentiation: Emphasize the safety profile and long-term data supporting diuretic use.
- Pricing Strategies: Leverage cost advantages of the generic market while maintaining value propositions.
- Engagement: Strengthen relationships with prescribing physicians through continued education on monotherapy benefits and adherence improvement.
- Portfolio Expansion: Explore formulations with enhanced dosing flexibility or adjunct therapies to maintain relevance.
Key Takeaways
- Stable Positioning: DYAZIDE maintains relevance in a mature antihypertensive market, with sales driven primarily by volume in a highly commoditized environment.
- Growth Moderation: The expiration of patent exclusivity and increasing generic competition are likely to cap revenue growth, emphasizing the importance of volume management and formulary access.
- Market Expansion: Opportunities exist in institutional settings and among elderly populations, provided strategic engagement with payers and prescribers.
- Competitive Edge: Emphasizing long-term safety, affordability, and proven efficacy can sustain market share amidst rising competition.
- Monitoring Trends: Incorporating emerging therapies and regulatory developments is vital for adapting sales strategies and maximizing revenue potential.
FAQs
1. How does patent expiry impact DYAZIDE sales?
Patent expiration introduces multiple generic competitors, leading to significant price erosion and increased market share for generics, thus reducing branded revenue. However, effective marketing and formulary positioning can mitigate this impact.
2. What patient populations most benefit from DYAZIDE?
Elderly hypertensive and edematous patients who require effective diuretic therapy with potassium-sparing benefits are primary beneficiaries. The safety profile favors long-term management in chronic conditions.
3. Are there emerging therapies threatening DYAZIDE’s market?
Yes. Newer antihypertensive agents, including novel classes like ARNIs and SGLT2 inhibitors, may gradually displace traditional diuretics in some clinical scenarios, especially where cardiovascular benefits are established.
4. What strategies can enhance DYAZIDE’s market share?
Securing formulary approval, emphasizing cost-effectiveness, engaging with prescribers through education, and expanding indications or formulations are key strategies.
5. How will regulatory changes affect DYAZIDE’s future sales?
Potential policies aiming to control drug prices or favor biosimilars could exert downward pressure on revenues, necessitating proactive market access strategies and cost management.
References
[1] MarketWatch. (2023). “Global antihypertensive drugs market outlook.”
[2] CDC. (2022). “Hypertension prevalence among US adults.”
[3] FDA. (2022). “Generic drug approvals and patent statuses.”
[4] FDA. (2023). “Biosimilar and generic drug policies.”
[5] ACC/AHA. (2017). “High Blood Pressure Clinical Guidelines.”
More… ↓
