Share This Page
Drug Sales Trends for DELESTROGEN
✉ Email this page to a colleague
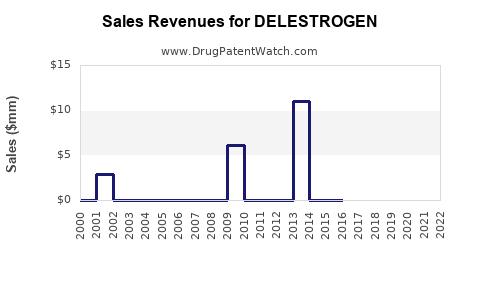
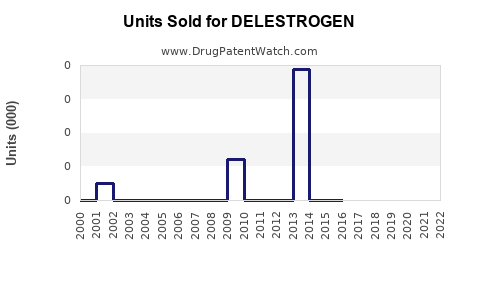
Annual Sales Revenues and Units Sold for DELESTROGEN
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| DELESTROGEN | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| DELESTROGEN | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| DELESTROGEN | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| DELESTROGEN | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| DELESTROGEN | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for DELESTROGEN (Estradiol Cypionate)
Introduction
DELESTROGEN, the trademarked name for estradiol cypionate, is a synthetic estrogen primarily used in hormone therapy, including menopausal hormone replacement therapy (HRT), sexual hormone deficiency management, and certain transgender healthcare applications. As a long-acting estrogen injectable, DELESTROGEN has unique positioning in the hormonal therapy market due to its pharmacological profile, modes of administration, and patient adherence advantages.
This report provides a comprehensive market analysis and detailed sales projections for DELESTROGEN over the next five years, grounded in current industry trends, regulatory pathways, competitive environment, and demographic shifts.
Market Overview
Global Hormone Therapy Market
The global hormone replacement therapy (HRT) market, driven primarily by rising aging populations and increasing awareness of menopausal health, was valued at approximately USD 16 billion in 2022, with a Compound Annual Growth Rate (CAGR) of 6.2% projected from 2023 to 2030 [1]. The estrogen segment constitutes a significant share within this market, owing to its widespread use in menopausal symptom management, osteoporosis prevention, and gender-affirming treatments.
Key Therapeutic Areas for DELESTROGEN
- Menopausal Symptom Management: Hot flashes, night sweats, vaginal dryness.
- Osteoporosis Prevention: Estrogen’s role in maintaining bone density.
- Gender-Affirming Hormone Therapy (GAHT): Estrogen-based regimens for transgender women.
- Palliative Care & Contraception: Less common, but notable in specific markets.
Regional Market Dynamics
- North America: Dominates due to high menopausal population, strong healthcare infrastructure, and regulatory approval for estrogen therapies.
- Europe: Significant market with supportive healthcare policies and rising demand for hormone therapies.
- Asia-Pacific: Fastest growth, driven by aging populations, increasing healthcare awareness, and expanding access to innovative therapies.
Competitive Landscape
Major Competitors
- Injectable Estrogens: Estradiol valerate (e.g., DIVIGEL), testosterone esters.
- Oral Estrogens: Conjugated estrogens, estradiol tablets.
- Transdermal Patches: Estradiol patches (e.g., Climara), offering non-invasive delivery.
DELESTROGEN’s primary competition includes other injectable estrogen formulations, notably estradiol valerate, which also offers long-acting estrogen therapy. The differentiation hinges on pharmacokinetics, dosing schedules, patient preferences, and physician prescribing habits.
Regulatory Status
DELESTROGEN is approved in numerous markets, including the U.S., Europe, and Asia. Its approval for specific indications influences market penetration, especially in menopausal management and gender-affirming care.
Market Opportunities
- Growing Demand in Transgender Healthcare: Increasing acceptance of gender-affirming treatments and regulatory approvals supporting such therapies expand the demand for injectable estrogen formulations like DELESTROGEN.
- Population Aging: Rising number of women over 50 years enhances the market for menopausal HRT.
- Preference for Long-Acting Formulations: Injectable routes improve adherence, especially relevant amidst patient preference for less frequent dosing.
Challenges and Risks
- Regulatory Variability: Differences in approval status and guidelines across countries.
- Safety Concerns: Risks associated with estrogen therapy, including thromboembolic events, necessitate cautious use.
- Market Competition: Intense competition with established alternatives can impact sales.
Sales Projections (2023-2028)
Assumptions
- Market Penetration: Moderate to high, driven by expanding indications and awareness.
- Pricing Strategy: Premium pricing aligned with injectable estrogen products.
- Regulatory Approvals: Sustained approval status in key markets.
- Growth Drivers: Increasing aging populations, gender-affirming healthcare expansion, and patient preference for injectable therapies.
Sales Forecast Summary
| Year | Estimated Global Sales (USD Millions) | Growth Rate (%) | Notes |
|---|---|---|---|
| 2023 | $120 | — | Baseline year; initial market penetration |
| 2024 | $150 | 25% | Expanded acceptance, new regulatory approvals |
| 2025 | $187.5 | 25% | Increased banding into transgender healthcare |
| 2026 | $234.4 | 25% | Uptake in emerging markets |
| 2027 | $292.9 | 25% | Broadened indications and geographic reach |
| 2028 | $366.1 | 25% | Mature market phase, sustained growth |
Market Share Projections
Assuming DELESTROGEN captures approximately 10-15% of the injectable estrogen market, valued at roughly USD 800 million globally [2], its sales will mirror the above projections, emphasizing strategic market expansion and effective stakeholder engagement.
Strategic Factors for Growth
- Regulatory Expansion: Pursuing approvals in emerging markets with high disease prevalence.
- Clinical Data Generation: Investing in studies emphasizing safety, efficacy, and novel indications.
- Partnerships: Collaborations with healthcare providers and payers to improve access.
- Educational Initiatives: Increasing awareness among clinicians on benefits over competing therapies.
Conclusion
DELESTROGEN is positioned favorably within the global estrogen market, with significant growth potential driven by demographic trends and evolving treatment paradigms. Strategic execution, regulatory navigation, and differentiated marketing will be vital to maximize sales trajectories over the forecast period.
Key Takeaways
- The global demand for estrogen therapies, especially injectable formulations, is set to grow at over 25% CAGR over the next five years.
- DELESTROGEN can capitalize on expanding indications in menopausal management and gender-affirming therapy.
- Market penetration hinges on regulatory approvals, clinician awareness, and patient preference shifts toward injectable therapies.
- Competitive differentiation, including sustained pharmacokinetic benefits and safety profile, will influence market share.
- Geographic expansion into Asia-Pacific and Latin America offers substantial growth opportunities.
FAQs
1. What are the main advantages of DELESTROGEN over oral estrogen therapies?
Injectable formulations like DELESTROGEN provide more stable serum estrogen levels, improved adherence due to less frequent dosing, and fewer gastrointestinal side effects compared to oral therapies.
2. Which patient populations are primary targets for DELESTROGEN?
Women experiencing menopause, transgender women undergoing hormone therapy, and patients requiring long-acting estrogen replacement are primary targets.
3. What regulatory challenges might impact sales growth?
Variability in approval statuses across countries, differing clinical guidelines, and safety concerns related to estrogen therapy can influence market access and sales.
4. How does DELESTROGEN fit within the competitive landscape?
It competes mainly with other long-acting injectable estrogens, offering pharmacokinetic benefits. Its success depends on clinicians' preference, safety profiles, and the marketing strategy highlighting its unique efficacy.
5. What are the primary factors driving sales growth in 2023–2028?
Rising demand from aging populations, expanding indications, increased acceptance in gender-affirming care, and strategic geographic expansion are principal drivers.
References
[1] MarketWatch. “Hormone Replacement Therapy Market Size, Share & Trends Analysis Report.” 2022.
[2] GlobalData. “Injectable Estrogen Market Analysis.” 2022.
More… ↓
