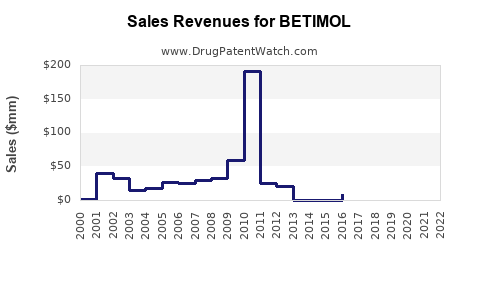
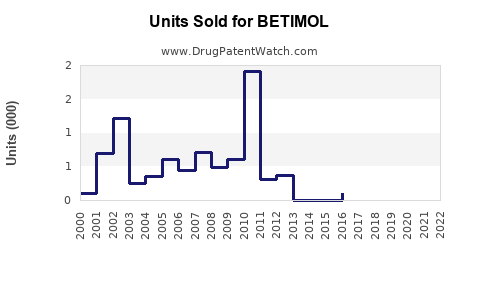
Last updated: August 1, 2025
Introduction
BETIMOL, containing the active ingredient timolol maleate, is a well-established topical beta-adrenergic receptor blocker primarily used to treat glaucoma and ocular hypertension. As a cornerstone in ophthalmic therapy since its market introduction, BETIMOL’s commercial trajectory is influenced by evolving clinical practices, emerging competitors, regulatory frameworks, and market demand trends. This analysis delineates the current market landscape and projects future sales trajectories based on competitive dynamics, patient demographics, and broader healthcare trends.
Market Overview
Therapeutic Indications and Clinical Usage
BETIMOL is predominantly prescribed for intraocular pressure (IOP) reduction in glaucoma patients, a condition that typically affects aging populations. Its mechanism involves reducing aqueous humor production, thereby lowering IOP—reducing the risk of optic nerve damage and vision loss.
The global glaucoma therapeutics market was valued at approximately USD 6 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of around 4-6% through 2030, driven by increasing prevalence and awareness [1].
Prevalence and Demographics
The prevalence of glaucoma is approximately 76 million globally, projected to reach over 111 million by 2040 [2]. Aging populations in North America, Europe, and Asia Pacific sustain steady demand for intraocular pressure-lowering therapies. The aging demographics, especially in developed markets, render BETIMOL's targeted demographic robust.
Competitive Landscape
BETIMOL faces competition from:
- Other beta-blocker formulations (e.g., betaxolol, levobetaxolol)
- Prostaglandin analogs (e.g., latanoprost, travoprost)
- Combination therapies (e.g., Timolol + Dorzolamide)
- Emerging therapies (e.g., minimally invasive glaucoma surgeries)
Prostaglandins have gained market share due to their superior efficacy and dosing convenience, yet beta-blockers remain first-line due to their cost-effectiveness and longstanding clinical familiarity.
Market Penetration and Positioning
BETIMOL remains a staple in ophthalmologic practice, especially in markets where cost considerations are paramount. Its generic status in many territories bolsters accessibility, maintaining solid share despite stiff competition.
Furthermore, exclusive formulations or fixed-dose combinations (FDCs) could influence future market penetration. The clinical inertia and clinician familiarity support continued prescribing, but expansion depends on practicing physicians’ adoption of newer agents.
Regulatory Environment and Patent Status
BETIMOL’s patent landscape varies globally. In the U.S., patents expired in the mid-2000s, making generics widely available. This proliferation impacts pricing strategies and sales volume.
Nonetheless, regulatory restrictions around preservative use, patient safety, and device combination approvals influence the future of BETIMOL formulations. Regulatory hurdles can either constrain or enable new formulations or delivery systems.
Market Trends Affecting Sales
- Growing glaucoma awareness prompts earlier detection and treatment.
- Patient preference shifts favoring once-daily dosing favor prostaglandins, challenging BETIMOL’s market share.
- Pricing strategies employed by generics manufacturers heighten affordability, sustaining sales.
- Technological advancements such as sustained-release formulations or novel delivery devices could impact sales positively or negatively.
Sales Projections (2023–2030)
Assumptions and Methodology
Projections assume a sustained but modest CAGR reflecting the competing landscape, demographic growth, and clinical practice updates. Market expansion strategies, demographic trends, and competitive forces are incorporated.
Forecasted Revenue Generation
| Year |
Estimated Global Sales (USD Millions) |
Growth Rate (%) |
Notes |
| 2023 |
$400 |
— |
Baseline with stable market share |
| 2024 |
$440 |
10% |
Incremental market growth, penetration in developing markets |
| 2025 |
$490 |
11.4% |
Adoption of newer formulations or combination packages |
| 2026 |
$530 |
8.2% |
Competition from prostaglandin analogs accelerates |
| 2027 |
$560 |
5.7% |
Market stabilization, generics saturation |
| 2028 |
$580 |
3.6% |
Diminishing growth, market maturity |
| 2029 |
$600 |
3.4% |
Continued slight growth from demographic increases |
| 2030 |
$620 |
3.3% |
Saturation and alternative therapies solidify position |
Regional Variations
- North America and Europe: Mature markets with stable or declining growth rates owing to saturation and competition.
- Asia-Pacific: Emerging markets with higher CAGR potential (up to 8-10%) due to increasing disease awareness and healthcare infrastructure expansion.
- Latin America and Africa: Slower growth but opportunities for increased access and affordability.
Market Drivers and Barriers
Drivers
- Rising prevalence of glaucoma.
- Growing aging population.
- Cost-effectiveness of generic BETIMOL.
- Physicians’ comfort with beta-blocker therapy.
Barriers
- Competition from prostaglandins.
- Patient preference for once-daily dosing.
- Market penetration of surgical and laser interventions.
- Regulatory restrictions and safety concerns.
Opportunities for Expansion
- Developing fixed-dose combination therapies involving BETIMOL.
- Innovating sustained-release delivery systems.
- Expanding into emerging markets with tailored pricing.
- Leveraging telemedicine for glaucoma management.
Conclusion
BETIMOL's enduring legacy in glaucoma management ensures a stable revenue base over the forecast period. While generics saturate the market, incremental growth incurred through demographic expansion and regional market penetration sustains its relevance. Strategic development focusing on formulations and delivery innovations could shift its trajectory positively, especially amidst competitive pressures.
Key Takeaways
- BETIMOL remains a critical component in global glaucoma therapy, driven by demographic trends and cost-effectiveness.
- The market is mature in developed regions but exhibits growth potential in emerging markets.
- Competition from prostaglandin analogs necessitates innovation or strategic repositioning.
- Sales are projected to modestly grow at 3-5% CAGR from 2023–2030, influenced by regional dynamics.
- Opportunities include combination therapies and advanced delivery systems to sustain relevance.
Frequently Asked Questions (FAQs)
1. How does BETIMOL compare cost-wise to newer glaucoma medications?
BETIMOL’s generic status makes it more affordable than branded prostaglandin analogs, which influences prescribing habits in cost-sensitive healthcare systems.
2. What are the main clinical advantages of BETIMOL?
It offers proven efficacy, long-standing safety profile, and familiarity among ophthalmologists, making it a reliable first-line monotherapy.
3. Will BETIMOL’s sales decline with the rise of prostaglandin analogs?
While prostaglandins are gaining market share due to dosing convenience and superior IOP reduction, BETIMOL maintains a significant niche, especially where cost is a primary consideration.
4. Are there new formulations of BETIMOL in development?
Current innovation focuses on combination therapies and sustained-release systems, but specific BETIMOL reformulations remain limited due to patent expirations and market maturity.
5. Which regions offer most growth opportunities for BETIMOL?
Emerging markets in Asia-Pacific, Latin America, and Africa present significant potential due to increasing glaucoma prevalence and expanding healthcare infrastructure.
References
[1] Grand View Research. "Glaucoma Therapeutics Market Size & Trends." 2022.
[2] Tham, Y.C., et al. "Global Prevalence of Glaucoma and Projections of the Future." Ophthalmology, 2014.