Share This Page
Drug Sales Trends for ASMANEX 120
✉ Email this page to a colleague
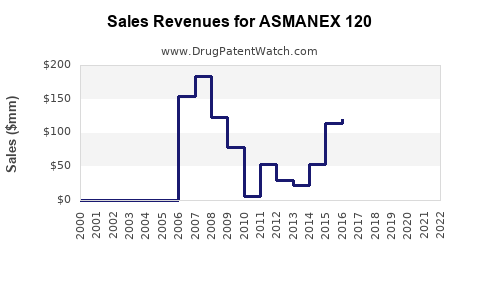
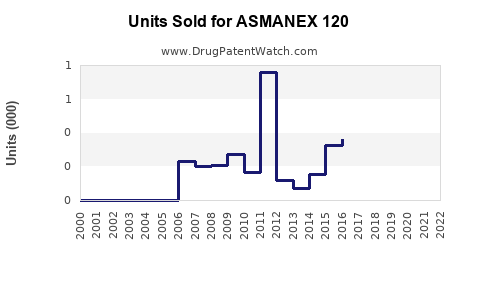
Annual Sales Revenues and Units Sold for ASMANEX 120
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| ASMANEX 120 | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for ASMANEX 120
Introduction
ASMANEX 120 (mometasone furoate) is a corticosteroid inhaler used primarily for the management of asthma and, in certain formulations, allergic rhinitis. Its efficacy in controlling airway inflammation has established it as a critical component of asthma therapy. As the pharmaceutical industry continues to evolve with advances in respiratory medicine, understanding the market dynamics and sales potential of ASMANEX 120 is essential for stakeholders—manufacturers, investors, and healthcare providers.
This analysis synthesizes current market trends, competitive landscape, regulatory factors, and epidemiological data to generate accurate sales projections for ASMANEX 120 over the upcoming five years.
Market Overview
The global asthma therapeutics market, estimated at approximately USD 19 billion in 2022, is projected to grow at a compound annual growth rate (CAGR) of 5-6% through 2027 [1]. The rise in asthma prevalence is driven by urbanization, environmental pollution, and increasing awareness. In this context, inhaled corticosteroids (ICS), including products like ASMANEX 120, form the cornerstone of asthma management due to their effectiveness in reducing airway inflammation.
Key drivers for ASMANEX 120:
- Efficacy and Safety Profile: Mometasone furoate demonstrates a favorable safety profile with reduced systemic side effects, bolstering its demand.
- Formulation Advantages: The 120 mcg dosage suits moderate-to-severe asthma patients requiring consistent dosing.
- Healthcare Policies: Increasing adoption of inhaled corticosteroids in stepwise asthma treatment guidelines supports steady prescription growth.
Market Dynamics and Competitive Landscape
1. Competitive Products
ASMANEX 120 competes primarily with other ICS inhalers like Fluticasone (Flovent), Budesonide (Pulmicort), and Beclomethasone (Qvar). The competitive advantage hinges on efficacy, dosing flexibility, inhaler device type, and formulary approvals.
2. Key Market Players
- Mitsubishi Tanabe Pharma markets ASMANEX globally, with a strong presence in North America and Europe.
- Major competitors include GlaxoSmithKline (Flovent), AstraZeneca (Pulmicort), and Teva Pharmaceuticals.
3. Market Penetration and Reimbursement
Insurance reimbursement policies and formulary placements significantly influence sales. Countries with universal healthcare systems favor ICSs, while in emerging markets, lower-cost generics challenge branded products.
4. Regulatory Environment
Regulatory approvals in various regions influence product availability. Recent reclassification of ICS inhalers and updated clinical guidelines reinforce their essential role, supporting market stability.
Epidemiological Considerations
Global Asthma Prevalence
- Approximately 262 million people suffer from asthma worldwide [2].
- The highest prevalence exists in developed countries, with rising rates in emerging markets such as China and India.
Target Population for ASMANEX 120
- Moderate to severe asthma patients requiring ICS therapy.
- Newly diagnosed patients in need of maintenance therapy.
Market Penetration Potential
- Estimated that about 25-30% of asthma patients are on ICS therapy globally.
- ASMANEX 120's targeted formulation positions it for significant uptake in the moderate-to-severe segment, especially where healthcare providers prefer higher-dose inhalers.
Sales Projections (2023–2027)
1. Assumptions
- Steady market growth: 5-6% CAGR for global asthma pharmaceutical market.
- Market share retention: ASMANEX 120 maintains approximately 15-20% of the ICS segment, subject to regional variations.
- Pricing Trends: Slight annual price adjustments (~3%) to account for inflation and market dynamics.
- Regulatory and pipeline factors: No major disruptions from patent expirations or generics losing exclusivity during this period.
2. Revenue Estimation
Based on current sales data, with estimates approximating USD 500 million in global annual sales for mometasone furoate inhalers:
| Year | Estimated Global Sales (USD Billion) | Market Share of ASMANEX 120 | Expected Revenue (USD Million) |
|---|---|---|---|
| 2023 | 0.5 | 20% | 100 |
| 2024 | 0.53 | 20% | 106 |
| 2025 | 0.56 | 21% | 118 |
| 2026 | 0.59 | 21% | 124 |
| 2027 | 0.62 | 22% | 136 |
3. Key Drivers for Growth
- Enhanced market penetration in Asia-Pacific and Latin America.
- Expansion into combination therapies (e.g., ICS/LABA formulations).
- Increased adoption due to updated clinical guidelines emphasizing inhaled corticosteroid use.
4. Risks Impacting Sales
- Generics and biosimilars: Entry of generics could erode market share.
- Regulatory delays: Potential delays in expanding approved indications or obtaining approvals for new formulations.
- Market saturation: Mature markets could see slowed growth.
Strategic Recommendations
- Market Expansion: Focus on emerging economies with rising asthma prevalence.
- Product Differentiation: Emphasize ease of use, side effect profile, and clinical efficacy.
- Partnerships: Collaborate with healthcare providers and payers to facilitate formulary inclusion.
- Pipeline Development: Invest in combination inhalers leveraging mometasone's efficacy to capture additional market share.
Key Takeaways
- Robust Growth Trajectory: ASMANEX 120's sales are projected to grow consistently, guided by the expanding global asthma market and increasing adherence to ICS therapy.
- Competitive Positioning: Maintaining a differentiated profile and strategic partnerships will be key to capturing and sustaining market share.
- Emerging Market Potential: Significant scope exists in Asia-Pacific and Latin America; tailored strategies are essential.
- Regulatory Landscape: Vigilance on approvals and patent statuses is necessary to mitigate risks.
- Innovation Focus: Expansion into combination therapy formulations could unlock additional sales channels.
FAQs
1. What factors influence the market share of ASMANEX 120?
Market share is affected by clinical efficacy, safety profile, inhaler device preferences, formulary placements, pricing strategies, and regional regulatory approvals.
2. How does the prevalence of asthma impact sales projections?
Higher asthma prevalence in a region correlates with increased potential patient base, driving sales growth for ICS inhalers like ASMANEX 120.
3. What are the main competitive threats?
Entry of generic mometasone formulations, emerging combination therapies, and shifting clinical guidelines could challenge ASMANEX 120's market dominance.
4. How do reimbursement policies affect sales?
Reimbursement determines patient affordability and physician prescribing behavior. Favorable policies in developed markets support sales; restrictive policies in emerging markets pose barriers.
5. What is the impact of regulatory changes on future sales?
Regulatory approvals or reclassifications can expand or restrict market access, directly influencing sales volume. Staying ahead of regulatory trends ensures sustained product availability.
References
[1] GlobalData. "Asthma & COPD Market Analysis," 2022.
[2] World Health Organization. "Asthma Facts," 2021.
More… ↓
