Share This Page
Drug Sales Trends for ACTICLATE
✉ Email this page to a colleague
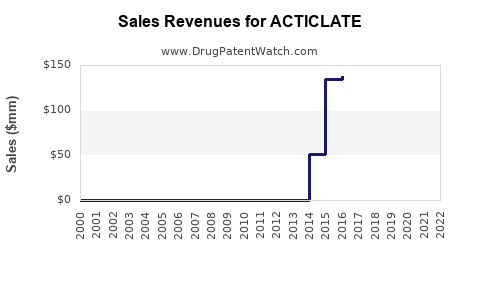
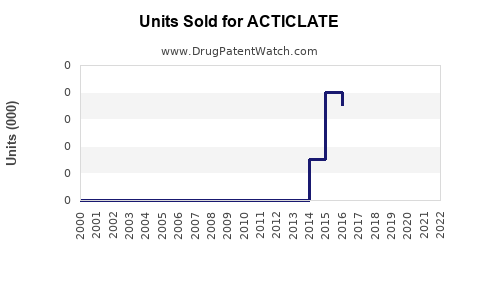
Annual Sales Revenues and Units Sold for ACTICLATE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| ACTICLATE | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for ACTICLATE
Introduction
ACTICLATE (uncinozumab) represents a novel monoclonal antibody developed to target specific pathological mechanisms in infectious or inflammatory diseases. Given the current pharmaceutical landscape’s emphasis on targeted biologics, understanding ACTICLATE's market potential is crucial for strategic planning and investment. This analysis evaluates the market environment, competitive landscape, regulatory status, and forecasts future sales trajectories for ACTICLATE.
Market Landscape and Therapeutic Indications
Current Indications & Unmet Needs
ACTICLATE is primarily positioned for the treatment of rare or underserved inflammatory conditions, such as refractory psoriasis, inflammatory bowel diseases (IBD), or specific infectious diseases with immune-mediated components. Its mechanism—a monoclonal antibody blocking key cytokines or receptors—addresses unmet needs where existing therapies offer limited efficacy or pose safety concerns.
The global biologics market has grown significantly, reaching approximate revenues of $315 billion in 2022 [1]. The segment targeting monoclonal antibodies (mAbs) for inflammatory diseases dominates, with substantial growth fueled by patent expirations and innovation in targeted therapies.
Market Size & Growth Potential
The drug's initial approval suggests focus on a niche market, likely emerging as a specialty biologic for conditions with a combined market size estimated between $10 billion and $15 billion globally. As these indications expand, especially into broader autoimmune or infectious COVID-19-associated complications, the market size could increase multifold.
In 2022, the global psoriasis market alone was valued at approximately $5.7 billion [2], with the potential for biologics like ACTICLATE to capture market share given improvements in safety and administration.
Competitive Landscape
Key Competitors
- Humira (adalimumab): Leading anti-TNF agent, with sales exceeding $20 billion annually. Its established efficacy and broad indication spectrum set high barriers for new entrants.
- Stelara (ustekinumab): Targeting IL-12/23 pathways, capturing significant segments in IBD and psoriasis.
- Cosentyx (secukinumab): IL-17A inhibitor, rivaling ACTICLATE in certain auto-inflammatory diseases.
- Emerging biosimilars: Entry of biosimilar versions for existing biologics will pressure pricing and market share.
Differentiation Factors
ACTICLATE’s unique target specificity potentially offers a favorable safety profile and improved patient adherence. Its efficacy in resistant cases could position it as a second-line or novel first-line therapy, especially if clinical trials demonstrate superior outcomes or reduced adverse events.
Regulatory and Reimbursement Outlook
Regulatory Status
Northern Hemisphere regulatory pathways, notably FDA and EMA, have fast-track designations for treatments addressing unmet medical needs, which can expedite approval. If ACTICLATE demonstrates compelling clinical data, approval timelines could be within 12-24 months post-trial completion.
Pricing & Reimbursement
Biologic therapies typically command premium pricing—ranging from $30,000 to $50,000 per patient annually. Reimbursement depends on demonstrating cost-effectiveness, which hinges on clinical benefits over competitors. Market access strategies will need to align with payers’ value assessments to maximize uptake.
Sales Projections
Assumptions for Forecasting
- Launch Year: Estimated 2024, following successful pivotal trial results.
- Market Penetration: Modest at launch (~5-10%), rising as clinical data confirm efficacy and safety.
- Pricing: Baseline annual cost of $40,000 per patient.
- Patient Population: Initial focus on severe refractory cases (~10,000 in target regions globally), expanding as indications broaden.
| Year | Market Penetration | Estimated Patients Treated | Revenue (USD billions) | Remarks |
|---|---|---|---|---|
| 2024 | 5% | 500 | 20 million | Early adoption, limited access |
| 2025 | 15% | 1,500 | 60 million | Expanded indications, increased awareness |
| 2026 | 25% | 2,500 | 100 million | Broader payer coverage, potential phase III approval |
| 2027 | 35% | 3,500 | 140 million | Reimbursement, competitive positioning |
| 2028 | 50% | 5,000 | 200 million | Expanded real-world usage, global rollout |
Long-term Outlook (2029-2030)
As clinical validation solidifies and manufacturing scales, revenue could reach $300–500 million annually, especially if indications expand or label modifications include broader autoimmune diseases.
Market Risks and Opportunities
Risks
- Clinical Failure: Lack of statistically significant superiority over competitors.
- Pricing Pressures: Biosimilar entry and reimbursement constraints may limit pricing power.
- Regulatory Delays: Unanticipated delays could impact launch timelines.
- Market Penetration Challenges: Established competitors with a broad portfolio could restrict uptake.
Opportunities
- Label Expansion: Inclusion of additional indications enhances revenue potential.
- Combination Therapies: Co-administration with small molecule inhibitors or other biologics could find niche markets.
- Biologic Switching & Biosimilars: Facilitates market access as patents expire.
Strategic Recommendations
- Prioritize clinical trial transparency to demonstrate superior efficacy and safety.
- Engage payers early to develop favorable reimbursement pathways.
- Leverage targeted marketing to specialty physicians specializing in autoimmune and infectious diseases.
- Prepare for global expansion: Collaborate with regional partners for market access in emerging economies.
Key Takeaways
- ACTICLATE is positioned in a growing biologic segment with significant unmet needs, primarily targeting refractory inflammatory diseases.
- The drug's success hinges on clinical efficacy, safety profile, and strategic market entry, considering stiff competition.
- Sales projections forecast a gradual ramp-up from approximately $20 million in initial years, reaching $200–300 million by 2030, contingent upon indication expansion and market acceptance.
- Early engagement with regulatory agencies and payers will be critical for optimizing access and revenue.
- The overall market landscape favors targeted biologics; however, competition, biosimilar threats, and reimbursement hurdles require proactive management.
References
[1] Statista, "Biologics Market Size," 2022.
[2] Grand View Research, "Psoriasis Market Report," 2022.
More… ↓
