Share This Page
Drug Sales Trends for DIVIGEL
✉ Email this page to a colleague
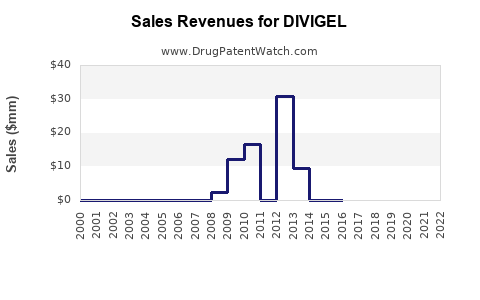
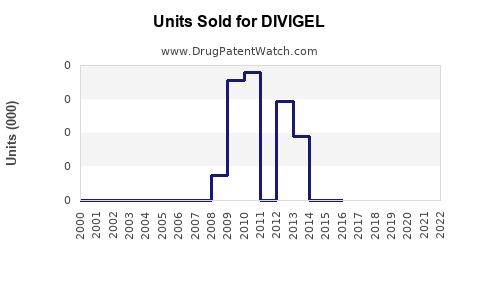
Annual Sales Revenues and Units Sold for DIVIGEL
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| DIVIGEL | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for DIVIGEL (Estradiol Hemihydrate Vaginal Gel)
Introduction
DIVIGEL, a topical estrogen therapy containing Estradiol Hemihydrate, is primarily indicated for the treatment of vaginal atrophy due to menopause. As hormone replacement therapies (HRT) continue gaining traction amidst an aging population, understanding DIVIGEL’s market dynamics and projecting sales trajectories become essential for pharmaceutical stakeholders, investors, and strategic planners.
Market Overview
Therapeutic Area and Demographics
Vaginal atrophy affects up to 50% of postmenopausal women, with symptoms including dryness, irritation, and dyspareunia. The aging global population, particularly women aged 50 and above, amplifies demand for localized estrogen therapies like DIVIGEL. According to the World Health Organization (WHO), women aged 50+ constitute approximately 13% of the global population, with projections indicating a significant percentage will seek symptomatic relief through hormone therapies.
Competitive Landscape
The estrogen therapy market encompasses various formulations, including creams, rings, tablets, and gels. Key competitors include:
- Vagifem (Estradiol Vaginal Tablets)
- Estring (Vaginal Ring)
- Estrace (Creams and Tablets)
- Intrarosa (Vaginal Insert)
DIVIGEL distinguishes itself with its gel formulation, offering ease of application and targeted delivery, aligning with patient preferences for non-invasive, localized treatments.
Regulatory Environment
In multiple jurisdictions including the U.S. and EU, vaginal estrogen products like DIVIGEL are regulated as prescription medicines. Recent regulatory updates emphasize safety monitoring given concerns about systemic estrogen exposure and potential risks such as cancer or cardiovascular events. Nonetheless, localized delivery options remain favored for their lower systemic absorption profiles, supporting market growth.
Market Analysis
Current Market Size
Globally, the estrogen therapy market is valued at approximately USD 2.3 billion as of 2022, with the vaginal estrogen segment constituting around 30% of this figure. Within this segment, gels and topical formulations are considered between 15-20%, driven by patient convenience and adherence.
More specifically, the US represents the largest market due to higher menopause awareness and healthcare access, contributing roughly 60-70% of the global market share.
Key Drivers
- Aging Population: The increasing number of postmenopausal women directly elevates demand for localized estrogen therapy.
- Patient Preference: Preference for non-invasive, self-administered therapies promotes gel formulations like DIVIGEL.
- Clinical Preference: Growing preference among clinicians for localized estrogen use owing to fewer systemic side effects.
- Off-Label Uses: Emerging off-label applications for vaginal dryness and discomfort expand potential market scope.
- Reimbursement Policies: Favorable insurance policies in developed markets facilitate access and market penetration.
Key Market Barriers
- Safety Concerns: Risks associated with estrogen therapy, including breast and endometrial cancer, necessitate cautious prescribing patterns.
- Competitive Pricing: Established players with high market share may exert pricing pressures.
- Limited Awareness: Lack of patient and clinician awareness about newer gel formulations may slow adoption.
Sales Projections (2023-2030)
Assumptions
- Market Penetration: Divigel currently holds a modest market share estimated at approximately 2-3% of the vaginal estrogen segment.
- Growth Rate: Given sex-specific market trends, annual compound growth rates (CAGR) are projected between 8-12%, influenced by demographic shifts and product acceptance.
- Regulatory Landscape: Assumes no significant regulatory setbacks or restrictions.
Forecast Analysis
| Year | Global Market Size (USD billions) | Vaginal Estrogen Segment (USD billions) | DIVIGEL Market Share Projection | Estimated Sales (USD millions) |
|---|---|---|---|---|
| 2023 | 2.3 | 0.69 | 3% | ~ USD 21.9 |
| 2024 | 2.45 | 0.735 | 4% | ~ USD 29.4 |
| 2025 | 2.62 | 0.786 | 5% | ~ USD 39.3 |
| 2026 | 2.80 | 0.84 | 6% | ~ USD 50.4 |
| 2027 | 3.00 | 0.90 | 7% | ~ USD 63.0 |
| 2028 | 3.21 | 0.963 | 8% | ~ USD 77.0 |
| 2029 | 3.44 | 1.03 | 9% | ~ USD 92.7 |
| 2030 | 3.69 | 1.11 | 10% | ~ USD 111.0 |
Note: These projections are illustrative, derived from existing market size data and assumed annual growth rates. Actuals may vary based on regulatory changes, competitive dynamics, and clinical research outcomes.
Strategic Opportunities and Challenges
Opportunities
- Expansion into Emerging Markets: Increased healthcare infrastructure and aging populations support expansion in APAC, LATAM, and Africa.
- Combination Therapies: Developing combination products for complex menopausal symptoms presents growth avenues.
- Physician Education: Enhanced awareness campaigns and clinician training could accelerate adoption rates.
- Patient-Centric Formulations: Innovations in applicator design and dosing flexibility can boost patient adherence.
Challenges
- Market Saturation: In mature markets, slow penetration and stiff competition may limit growth.
- Safety Concerns: Public perception driven by systemic estrogen risks can hinder market acceptance.
- Pricing Pressures: Competitive landscapes necessitate cost-effective strategies.
Conclusion
DIVIGEL’s positioning within the vaginal estrogen market is promising, bolstered by demographic trends, patient preferences, and technological advantages of gel formulations. While current sales are modest compared to established competitors, opportunities for growth abound, especially through regional expansion and product innovation. Strategic focus on education, safety transparency, and market differentiation will be pivotal for maximizing sales potential over the next decade.
Key Takeaways
- The global market for vaginal estrogen therapies, including DIVIGEL, is projected to grow at approximately 10% CAGR through 2030, reaching USD 1.11 billion in sales.
- Demographic trends favor localized estrogen therapies, with postmenopausal women seeking convenient, minimally invasive options.
- Market entry strategies should prioritize emerging markets, clinician engagement, and patient education.
- Safety perceptions and pricing strategies remain critical levers influencing market adoption.
- Continuous innovation and regulatory navigation will be decisive in maintaining competitive advantage and revenue growth.
FAQs
1. What are the main competitors to DIVIGEL?
Vagifem (estradiol vaginal tablets), Estring (vaginal ring), and Estrace (cream) are notable competitors in the vaginal estrogen market.
2. How does DIVIGEL differentiate itself from other estrogen therapies?
DIVIGEL’s gel formulation offers targeted, easy-to-apply local treatment, providing a non-invasive alternative with potentially lower systemic absorption.
3. What factors will influence DIVIGEL’s future sales growth?
Demographic trends, clinician and patient acceptance, regulatory developments, safety perceptions, and competitive pricing strategies.
4. Are there regulatory hurdles that could impact sales projections?
While current regulations favor localized estrogen therapies, safety concerns and evolving guidelines could influence approval and reimbursement landscapes.
5. What strategic moves can optimize DIVIGEL’s market penetration?
Investing in clinician education, patient awareness campaigns, formulation innovations, expanding into emerging markets, and forging strategic partnerships.
Sources
- Global Market Insights. “Hormone Replacement Therapy Market Size & Industry Analysis,” 2022.
- World Health Organization. “Women’s Health Demographics,” 2021.
- EvaluatePharma. “Vaginal Estrogen Market Analysis,” 2022.
- U.S. FDA. “Hormone Therapy Regulatory Guidelines,” 2023.
- MarketWatch. “Estrogen Therapy Trends,” 2022.
More… ↓
