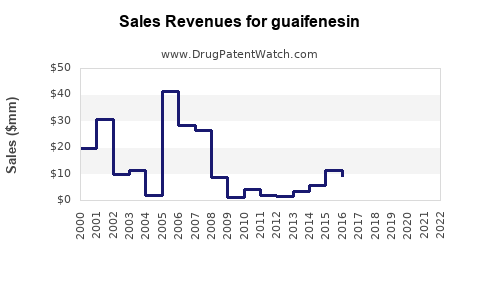
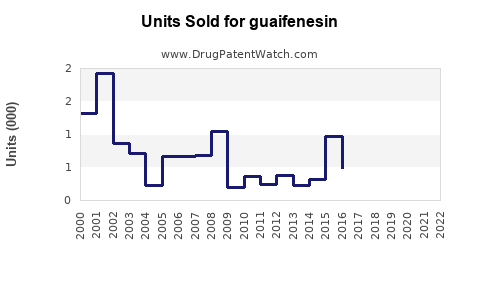
Last updated: July 28, 2025
Introduction
Guaifenesin, a widely used expectorant, has maintained a significant presence in OTC therapeutics primarily for symptomatic relief of cough and chest congestion. Its global market landscape reflects evolving consumer preferences, regulatory frameworks, and competitive dynamics. Understanding its current market positioning and future sales trajectory enables pharmaceutical companies and investors to make strategic decisions regarding research, manufacturing, and marketing investments.
Market Overview
Historical Market Context
First introduced in the 1950s, guaifenesin initially gained OTC recognition for its efficacy in loosening phlegm. Its longstanding utility in respiratory remedy formulations facilitated widespread adoption. The compound’s favorable safety profile, minimal side effects, and over-the-counter (OTC) availability have cemented its role in cold and cough treatment markets (1).
Global Market Size
The global expectorants market was valued at approximately USD 1.5 billion in 2022, with guaifenesin accounting for a significant portion, roughly 70%, due to its predominant use in combination products and standalone formulations (2). The North American region dominates sales due to high OTC consumption and advanced healthcare infrastructure, followed by Europe and Asia-Pacific.
Key Drivers
- Rising Incidence of Respiratory Diseases: Increasing prevalence of cough, cold, and respiratory ailments globally fuels demand.
- Over-the-Counter Accessibility: Regulatory ease promotes OTC sales.
- Consumer Preference for Familiar Remedies: Established safety profile encourages continued use.
- Combination Therapies: Many formulations combine guaifenesin with other active ingredients, expanding its reach.
Regulatory Trends
The regulatory environment remains generally favorable for guaifenesin as an OTC medication, with agencies like the FDA in the U.S. classifying it as Generally Recognized As Safe (GRAS) (3). However, evolving regulations, such as restrictions on certain dosage forms or concentration limits, could influence market dynamics.
Market Segmentation and Competitive Landscape
Product Segments
- Standalone Guaifenesin Products: Syrups, tablets, and capsules.
- Combination Formulations: Products combining guaifenesin with dextromethorphan, pseudoephedrine, or acetaminophen.
Distribution Channels
- OTC retail outlets
- Pharmacies
- E-commerce platforms
- Hospitals and clinics (for prescriptions)
Major Players
Leading manufacturers include Johnson & Johnson, Reckitt Benckiser, and CVS Health, with a competitive advantage stemming from brand recognition, distribution reach, and formulation innovation.
Sales Projections (2023-2028)
Methodology and Assumptions
Forecasting efforts consider current market size, growth indicators, consumer behavior, and regulatory updates. Assumptions include moderate annual growth driven by increasing respiratory health awareness and sustained OTC consumption, with a compound annual growth rate (CAGR) estimated at 4% over the forecast period.
Projected Market Expansion
- 2023: USD 1.5 billion
- 2024: USD 1.56 billion
- 2025: USD 1.62 billion
- 2026: USD 1.68 billion
- 2027: USD 1.75 billion
- 2028: USD 1.82 billion
This projection reflects continued steady growth, with potential accelerants being increased respiratory illness prevalence (notably post-pandemic health trends) and expansion into emerging markets.
Regional Breakdown
- North America: Remains the largest market, with projected CAGR of 3.5%, driven by consumer preferences and OTC sales.
- Europe: Stable growth, approximately 3% CAGR, influenced by regulatory policies.
- Asia-Pacific: Expected to witness the highest growth (>6%), attributable to rising healthcare awareness, expanding OTC retail networks, and urbanization.
Market Trends and Innovations
Formulation Enhancements
Innovations focus on improved bioavailability, taste-masking for pediatric formulations, and combination products for multi-symptom relief. The emergence of fast-dissolving tablets and sugar-free syrups caters to diverse consumer needs.
Digital and E-commerce Growth
Increasing online availability broadens consumer access, especially in emerging markets. Digital marketing strategies further boost product visibility.
Regulatory and Health Policies
Emerging regulations on strength and combination product approvals can shape future formulations and marketing strategies.
Challenges and Opportunities
Challenges
- Stringent regulations, especially concerning combination therapies and concentration caps
- Competition from alternative expectorants and herbal remedies
- Consumer preference shifts toward holistic and natural products
Opportunities
- Expansion into emerging markets with rising healthcare expenditure
- Development of novel delivery systems enhancing compliance
- Strategic partnerships for product innovation and distribution
Key Takeaways
- Guaifenesin maintains a predominantly OTC status, facilitating consistent demand in developed markets.
- The global expectorants market is forecasted to grow at a CAGR of approximately 4% through 2028.
- North America remains the dominant market, while Asia-Pacific shows the highest growth potential.
- Product innovation focusing on patient compliance, taste profiles, and combination therapies will be critical.
- Regulatory developments could influence formulation standards and market access strategies.
FAQs
1. What factors are driving the increased demand for guaifenesin globally?
The rise in respiratory illnesses, consumer preference for OTC medications with proven safety, and increased awareness of cold and cough remedies fuel demand.
2. How might regulatory changes impact guaifenesin sales in the coming years?
Regulatory restrictions on dosage, combination products, or OTC classifications could limit sales or necessitate reformulation, impacting revenue streams.
3. Which regions offer the highest growth opportunities for guaifenesin?
Emerging markets in Asia-Pacific and Latin America present significant growth potential due to expanding healthcare infrastructure and rising disposable income.
4. What product innovations are anticipated in the guaifenesin market?
Developments include fast-dissolving formulations, sugar-free options, pediatric-friendly formulations, and advanced combination therapies.
5. How does the competitive landscape influence future sales projections?
High competition drives innovation and price competitiveness, potentially stabilizing or increasing market share for leading players, but regional entry barriers may pose challenges for new entrants.
References
- Pharmaceuticals Market Reports, GlobalData, 2022.
- Expectorants Market Size & Share, Grand View Research, 2023.
- FDA Regulations on Guaifenesin, U.S. Food and Drug Administration, 2022.