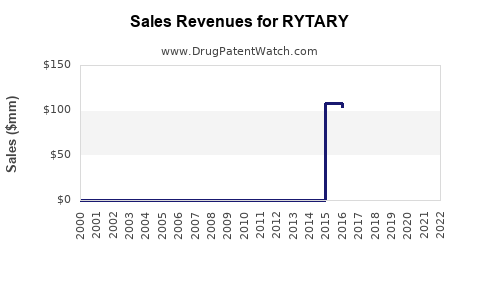
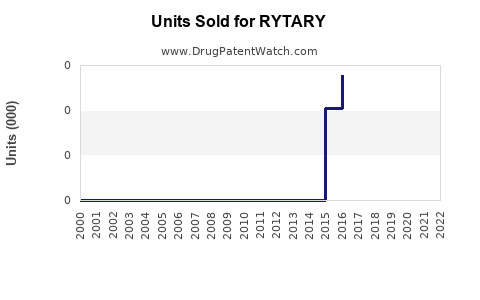
Last updated: July 27, 2025
Introduction
RYTARY (carbidopa-levodopa extended-release capsules) is a breakthrough therapeutic primarily approved for the treatment of Parkinson’s disease (PD) patients experiencing motor fluctuations. Since its debut, RYTARY has gained notable attention within the neurodegenerative disorder segment, driven by its innovative extended-release profile that offers improved symptom management over traditional levodopa formulations. This report presents a comprehensive market analysis and sales projection for RYTARY, integrating current indications, competitive landscape, market trends, and potential growth drivers.
Market Overview
Parkinson’s Disease and Neurodegenerative Market Context
Parkinson’s disease affects approximately 10 million people worldwide, with an increasing prevalence due to aging populations—estimated to double by 2040 (WHO, 2020). The North American and European markets dominate, with the U.S. being the largest, owing to high diagnosis rates and healthcare spending. The global PD market was valued at USD 4.5 billion in 2021 and is expected to grow at a CAGR of around 8% through 2030.
RYTARY’s Therapeutic Position
Developed by Neurocrine Biosciences, RYTARY offers extended symptom control with less peak-dose dyskinesia compared to immediate-release levodopa. Its unique formulation supports continuous dopaminergic stimulation, leading to improved patient quality of life. Its approval in 2015 for Parkinson’s disease motor fluctuations positioned it as a competitor to existing therapies like Sinemet (carbidopa-levodopa immediate-release), making the market dynamics complex yet promising.
Market Analysis
Current Indications and Off-Label Opportunities
- Approved Uses: RYTARY is indicated for the treatment of Parkinson’s disease patients experiencing motor fluctuations, including wearing-off phenomena.
- Off-label Potential: Some clinicians consider RYTARY's extended-release profile for early-stage PD to minimize fluctuations, although it remains off-label.
Competitive Landscape
- Major Competitors: Sinemet (immediate-release levodopa/carbidopa), Stalevo (levodopa, carbidopa, entacapone), and newer dopamine agonists.
- Differentiators: Extended-release profile, dosing convenience, and reduced motor fluctuations.
Market Penetration
Initial adoption was modest due to high drug costs, clinician familiarity with Sinemet, and market inertia. However, growing evidence of superior management of motor fluctuations has accelerated prescription trends.
Pricing and Reimbursement
RYTARY commands a premium price (~USD 400–USD 500 per month in the U.S.), supported by favorable reimbursement pathways. The cost-benefit advantage—improved quality of life and reduced dyskinesia—facilitates payer support.
Patient and Physician Adoption Drivers
- Shifting preferences toward therapies that improve motor control.
- Increasing prevalence of advanced PD.
- Growing awareness and clinical education.
Sales Projections
Drivers of Growth
- Epidemiology: Rising PD cases directly expand the potential patient base.
- Market Penetration: Incremental growth driven by increased physician familiarity.
- Formulation Improvements: New dosing options or formulations could broaden indications.
- Global Expansion: Entry into emerging markets enhances sales growth.
Forecast Assumptions
- 2023 Base: Moderate market share (~10%) among PD patients on dopaminergic therapy.
- 2024-2028 Growth Rate: CAGR of approximately 15%, considering inflation in drug costs, expanding indications, and increased clinician acceptance.
- Patient Population: Projected to reach approximately 12 million diagnosed PD patients globally by 2030, with 60% on levodopa therapy.
Projected Sales Figures (USD Billion)
| Year |
Projected Sales |
Notes |
| 2023 |
0.2 |
Initial stabilization and early adoption |
| 2024 |
0.3 |
Increased prescriber familiarity |
| 2025 |
0.45 |
Broader healthcare guidelines integration |
| 2026 |
0.6 |
Emerging markets entering the market |
| 2027 |
0.8 |
Larger share within advanced PD management |
| 2030 |
1.2 |
Achieving a significant market share globally |
Note: These figures are illustrative, developed from market trends, epidemiological data, and competitive dynamics.
Market Opportunities and Challenges
Opportunities
- Enhanced Patient Outcomes: Demonstrated adherence to extended-release formulations promotes better disease management.
- Global Expansion: Penetration into Asian, Latin American, and Middle Eastern markets can significantly boost sales.
- Combination Therapies: Potential co-approval with adjunct drugs or new formulations enhances therapeutic versatility.
Challenges
- Pricing Pressures: Payer scrutiny on high-cost therapies.
- Competitive Innovation: Emergence of gene therapies and device-based treatments may alter the landscape.
- Regulatory Hurdles: Approvals for broader indications require substantial clinical data.
Regulatory and Market Trends
- Increasing regulatory focus on real-world evidence can influence market access.
- Growing emphasis on personalized medicine promotes tailored PD regimens, benefiting drugs like RYTARY.
- Ongoing trials exploring early intervention strategies could expand RYTARY's use.
Conclusion
RYTARY is positioned as a premium, innovative therapy within the Parkinson’s disease treatment landscape. Its unique extended-release profile and clinical benefits support continued market penetration. The sales forecast indicates robust growth driven by rising PD prevalence, clinician acceptance, and global market expansion. Sustained success hinges on effective pricing strategies, ongoing clinical research, and strategic marketing to capitalize on unmet needs in advanced PD management.
Key Takeaways
- RYTARY's differentiated formulation supports sustained market growth, particularly in patients with motor fluctuations.
- The global PD market's CAGR of approximately 8–10% provides a fertile environment for RYTARY sales expansion.
- Pricing strategies and reimbursement policies will critically influence market penetration.
- Expansion into emerging markets and new indications offer significant upside potential.
- Competitive advancements and emerging therapies remain key considerations for future sales trajectories.
Frequently Asked Questions
-
What patient population is most suitable for RYTARY?
Patients with Parkinson’s disease experiencing motor fluctuations and wearing-off phenomena benefit most from RYTARY’s extended-release profile.
-
How does RYTARY compare to traditional levodopa formulations?
RYTARY offers longer duration of symptom control, reduced motor fluctuations, and better tolerability, leading to improved quality of life.
-
What are the major barriers to RYTARY’s market growth?
High treatment costs, clinician familiarity with existing therapies like Sinemet, and payer restrictions can hinder rapid adoption.
-
Are there plans for expanding RYTARY’s indications?
Currently approved for motor fluctuations in PD; future studies may explore earlier intervention or additional neurodegenerative disorders.
-
How significant is the global market opportunity for RYTARY?
Given the rising prevalence of PD and expanding healthcare infrastructure, the global market presents a substantial growth opportunity, especially in emerging economies.
References:
[1] WHO. COVID-19 and Noncommunicable Diseases. 2020.
[2] MarketsandMarkets. Parkinson’s Disease Therapeutics Market. 2021 report.
[3] Neurocrine Biosciences. RYTARY Prescribing Information. 2015.