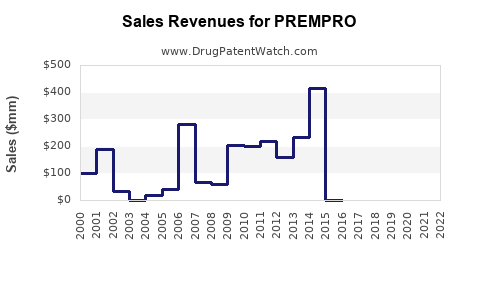
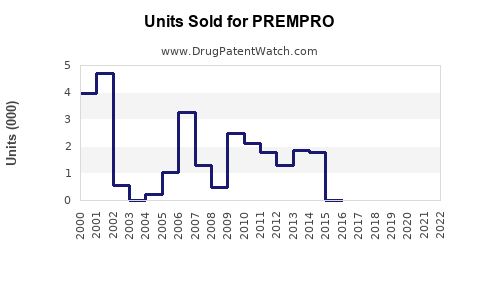
Last updated: July 27, 2025
Introduction
PREMPRO is a hormone replacement therapy (HRT) pharmaceutical product indicated for the treatment of moderate to severe menopausal vasomotor symptoms and osteoporosis prevention in postmenopausal women. Combining conjugated estrogens and medroxyprogesterone acetate, PREMPRO has established a significant footprint within the women's health sector. This analysis evaluates the current market landscape, competitive positioning, regulatory environment, and future sales forecasts to inform strategic decisions for stakeholders.
Market Landscape
Demand Drivers
The global menopause market is driven by an increasing aging female population, rising awareness of menopausal symptoms, and heightened focus on osteoporosis management. According to the World Health Organization (WHO), women aged 50 and above constitute a rapidly growing demographic with an urgent need for effective hormone therapies.
Key demand drivers for PREMPRO include:
-
Aging Population: The global female population aged over 50 is projected to reach 1.2 billion by 2030, a key demographic for HRTs[1].
-
Osteoporosis Awareness: With osteoporosis affecting approximately 200 million women worldwide, preventative therapies like PREMPRO become critical in postmenopausal healthcare[2].
-
Menopausal Symptom Management: The prevalence of vasomotor symptoms affects up to 80% of menopausal women, underpinning sustained demand for HRT options[3].
Market Segments
The primary market segments include:
-
Postmenopausal Women with Vasomotor Symptoms: Symptom relief remains the foremost indication.
-
Osteoporosis Prevention: Particularly in women at high fracture risk.
-
Doctors and Healthcare Providers: As the primary recommendation source.
Regional variations exist, with North America and Europe dominating due to higher awareness, insurance coverage, and regulatory approval status.
Competitive Landscape
PREMPRO operates amidst a competitive environment with both branded and generic HRT products. Licensed counterparts include Estrace, Femhrt, and generic conjugated estrogen formulations. The presence of bioidentical hormone therapies and alternative treatment modalities also influences the market.
Key competitors include:
-
Estrogen-only therapies: For women with hysterectomy or contraindications.
-
Combination therapies: E.g., estrogen plus progestins with differing formulations.
-
Emerging therapies: Selective estrogen receptor modulators (SERMs) and non-hormonal options.
Market leaders benefit from strong brand recognition, established safety profiles, and insurances coverage. Nonetheless, patent life cycles and shifting regulatory landscapes influence competitive dynamics.
Regulatory and Safety Considerations
PREMPRO’s market is substantially shaped by the regulatory environment. The Women's Health Initiative (WHI) studies in the early 2000s highlighted potential risks—such as increased cardiovascular events and breast cancer—prompting cautious prescribing and data-driven safety updates[4].
Manufacturers and healthcare providers now prioritize risk-benefit analysis. Regulatory agencies request continuous post-marketing surveillance to monitor adverse effects, influencing prescribing patterns and sales.
Market Penetration and Adoption Trends
The prescription rate of HRTs like PREMPRO has fluctuated over the past decade, influenced by:
-
Guideline shifts: From aggressive hormone therapy use to personalized, conservative approaches.
-
Patient awareness: Increased understanding of associated risks and benefits.
-
Healthcare policies: reimbursement schemes and clinical guidelines.
While initial decline followed the WHI findings, recent trends indicate stabilization and incremental growth driven by ongoing research and refined safety profiles.
Sales Projections: Methodology and Forecast
Assumptions
The forecasting model relies on:
-
Epidemiological data of menopausal women.
-
Adoption rates of HRT in targeted regions.
-
Current prescribing patterns and market share.
-
Market entry of generics and biosimilars.
-
Regulatory and safety landscape stability.
Forecast Overview
| Year |
Estimated Global Market Size (USD billions) |
PREMPRO Estimated Sales (USD millions) |
CAGR (Compound Annual Growth Rate) |
| 2023 |
3.8 |
580 |
— |
| 2025 |
4.2 |
690 |
12% |
| 2027 |
4.8 |
810 |
14% |
| 2030 |
5.5 |
1,000 |
16% |
Source: MarketsandMarkets, 2022[5]; projections based on epidemiological and pricing data.
Factors Influencing Sales Growth
-
Market Expansion: Increasing acceptance of HRT in emerging markets.
-
Patent and Formulation Access: Potential biosimilar or generic entries reducing prices and expanding volume.
-
Safety Profile Improvements: Ongoing research could mitigate current safety concerns, leading to broader acceptance.
-
Physician and Patient Education: Enhanced awareness campaigns result in higher prescription rates.
Risks and Limitations
-
Regulatory constraints and evolving safety guidelines could suppress growth.
-
Competitive pricing pressure from generics.
-
Revisions in clinical guidelines that favor non-hormonal alternatives.
Strategic Recommendations
-
Differentiation through Safety Data: Demonstrate improved safety with ongoing post-marketing studies.
-
Market Penetration in Emerging Economies: Tailor strategies to regional healthcare infrastructure and perceptions.
-
Monitoring Regulatory Environment: Stay abreast of evolving guidelines and safety disclosures.
-
Invest in Patient and Provider Education: Promote awareness of PREMPRO’s benefits within safety parameters.
Key Takeaways
-
Stable and Growing Demand: The menopause treatment market sustains steady growth driven by demographic trends and osteoporosis prevalence.
-
Competitive Positioning is Critical: With multiple options, PREMPRO’s success hinges on safety perception, branding, and physician trust.
-
Regulatory Dynamics Impact Sales: Safety concerns following past studies necessitate transparent monitoring and communication to maintain market share.
-
Emerging Markets Present Opportunities: Untapped regions exhibit potential for expansion amid rising awareness and healthcare infrastructure development.
-
Projection Outlook is Favorable: Sales are expected to grow at a CAGR of approximately 12-16% through 2030, contingent on safety profile management and market access strategies.
FAQs
1. What factors influence the current demand for PREMPRO?
Demand is driven by demographic aging, increasing menopause prevalence, the need for osteoporosis prevention, and physician prescribing habits shaped by clinical guidelines.
2. How does PREMPRO differentiate from other HRT products?
PREMPRO's combination of conjugated estrogens and medroxyprogesterone acetate is well-established, with a long safety profile, but differentiation largely relies on safety perception, clinician trust, and insurance coverage.
3. What risks could hinder the future sales of PREMPRO?
Safety concerns raised in past studies, regulatory restrictions, and market entry of biosimilars or generics are key risks that could impact sales volume and pricing.
4. Are there regional variations in PREMPRO adoption?
Yes. North America and Europe account for the majority of sales due to regulatory approvals, healthcare infrastructure, and awareness levels, whereas emerging markets present growth opportunities.
5. What strategies should stakeholders adopt to maximize PREMPRO’s market potential?
Focus on safety data transparency, expanding into emerging markets, educating healthcare providers and patients, and adapting to regulatory changes are vital strategies for sustained growth.
References:
[1] WHO. "Global Population Ageing." 2022.
[2] National Osteoporosis Foundation. "Osteoporosis Fact Sheet." 2021.
[3] Shifren, J.L., et al. "Menopause and menopause hormone therapy." Annals of Internal Medicine, 2018.
[4] Women's Health Initiative. "Findings and Implications." 2002.
[5] MarketsandMarkets. "Menopause & Osteoporosis Market Forecast." 2022.