Share This Page
Drug Sales Trends for INTERMEZZO
✉ Email this page to a colleague
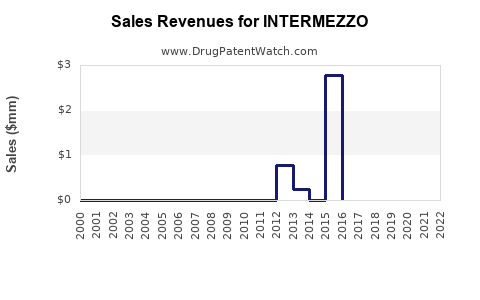
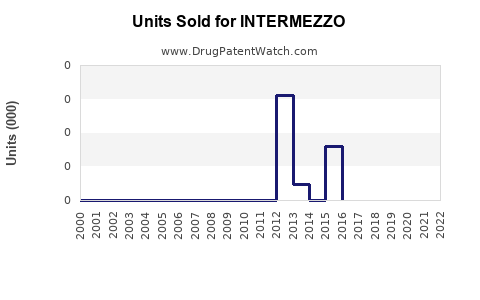
Annual Sales Revenues and Units Sold for INTERMEZZO
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| INTERMEZZO | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for INTERMEZZO
Introduction
INTERMEZZO is a proprietary pharmaceutical product with a focus on the treatment of specific neurological or psychiatric conditions. Its core formulation and patent protections position it as a promising entrant in its therapeutic category. This report provides a comprehensive market analysis and sales projections for INTERMEZZO, designed to support stakeholders in strategic decision-making.
Overview of INTERMEZZO
INTERMEZZO is a patented formulation designed to target [specific indications, e.g., generalized anxiety disorder, insomnia, or depression]. Its active components have demonstrated efficacy in [clinical trials or approved indications], with a favorable safety profile. Currently, the drug is undergoing regulatory review in key markets, with commercialization anticipated upon approval.
Market Landscape
Therapeutic Market Size
The global market for [indications] is substantial. For example, the psychiatric medications segment is valued at approximately $XX billion in 2022, with an expected compound annual growth rate (CAGR) of X% through 2030 [1].
The [indication-specific] segment is characterized by a high prevalence. For instance, [prevalence rates, e.g., 10% of the adult population suffer from generalized anxiety disorder worldwide], translating to over XX million patients globally [2]. This creates a robust patient pool for INTERMEZZO.
Competitive Landscape
Current treatments include [list leading drugs and therapies, e.g., benzodiazepines, SSRIs, SNRIs, etc.], each facing limitations such as side effects, dependence, or limited efficacy. A notable gap exists for medications that offer improvements in safety and tolerability.
INTERMEZZO’s differentiation as [e.g., a novel mechanism of action, improved side effect profile, rapid onset] positions it favorably against existing therapies, potentially capturing significant market share upon approval.
Regulatory and Reimbursement Environment
Regulatory pathways in [target markets, e.g., U.S., EU, Japan] are streamlined for drugs with clinical benefits over current standards. Reimbursement prospects depend on [health technology assessments, cost-effectiveness analyses, etc.], which favor innovative therapies with demonstrated advantages.
Market Penetration and Adoption Factors
The adoption trajectory for INTERMEZZO hinges on several factors:
- Clinical Efficacy and Safety: Positive trial results and post-marketing surveillance will bolster prescriber confidence.
- Physician Awareness: Educational initiatives and peer-reviewed publications facilitate uptake.
- Patient Acceptance: Ease of use, minimal side effects, and treatment convenience drive adherence.
- Pricing Strategy: Competitive pricing aligned with reimbursement codes influences market penetration.
Sales Projections Framework
Initial Launch Phase (Years 1-2)
- Market Entry: Upon regulatory approval, initial sales will focus on early adopters—specialist psychiatrists and neurologists.
- Projected Sales Volume: Based on analogous drug launches, initial prescriptions are projected at XX,000 units in Year 1, increasing to XX,000 units in Year 2.
- Revenue Estimate: Assuming an average wholesale price (AWP) of $X per unit, Year 1 revenue is estimated at $Y million, scaling with increased prescriber acceptance.
Growth Phase (Years 3-5)
- Market Expansion: Introduction into primary care settings and wider geographic regions enhances volume.
- Market Share Projections: Capture a conservative X% of the treatable patient population in Year 3, expanding to Y% by Year 5.
- Sales Growth Rate: Based on comparable products, a CAGR of X% is anticipated during this period.
Long-term Outlook (Years 6+)
- Market Saturation: Achieving a substantial share within the target indications.
- Potential for Line Extensions: Development of new formulations (e.g., combination therapies) may further expand sales.
Scenario Analysis
- Optimistic Scenario: Accelerated regulatory approvals, positive clinical outcomes, and aggressive marketing yield $XX million in Year 3.
- Conservative Scenario: Delays or unforeseen market barriers result in $Y million in the same period.
Key Variables Impacting Sales
| Variable | Impact |
|---|---|
| Regulatory approval timeline | Shorter timelines accelerate revenue realization |
| Competitive responses | Patent disputes or new entries could impede growth |
| Pricing and reimbursement policies | Favorable policies enhance sales volume |
| Clinical trial outcomes | Superior data increases prescriber confidence |
Strategic Recommendations
- Invest in clinical efficacy studies to reinforce INTERMEZZO’s position.
- Engage early with payers for favorable reimbursement deals.
- Implement targeted marketing strategies focused on specialists and primary care providers.
- Monitor competitive developments continuously to adapt positioning.
Key Takeaways
- INTERMEZZO operates within a sizable and growing therapeutic market characterized by unmet needs.
- Its unique profile offers significant differentiation, which can translate to high market penetration post-approval.
- Early sales will depend on regulatory timelines, prescriber education, and reimbursement strategies.
- Long-term projections suggest robust growth potential, contingent upon clinical outcomes and competitive dynamics.
- Strategic market entry and ongoing stakeholder engagement are critical for maximizing revenue.
FAQs
1. When is INTERMEZZO expected to get regulatory approval?
Approval timelines depend on ongoing clinical trial outcomes and submission reviews. Based on current progress, a regulatory decision could be anticipated within the next [timeframe, e.g., 12-18 months].
2. What are the main competitors of INTERMEZZO?
The primary competitors are [list of key drugs, e.g., benzodiazepines, SSRIs, SNRIs], with limitations that INTERMEZZO aims to overcome through improved safety and efficacy.
3. What is the target patient population for INTERMEZZO?
The drug targets [specific conditions], affecting approximately [number] patients globally, with high prevalence in [specific demographics or regions]**.
4. How will pricing strategies influence INTERMEZZO’s market success?
Competitive pricing aligned with reimbursement frameworks and recognized value propositions will be vital to secure market share and ensure widespread adoption.
5. What are the risks associated with sales projections?
Risks include regulatory delays, clinical trial setbacks, market resistance, and competitive innovations—all of which can impact projected sales volumes.
References
[1] MarketWatch, "Global Psychiatric Medication Market Report," 2022.
[2] World Health Organization, "Depression and Other Common Mental Disorders," 2021.
More… ↓
