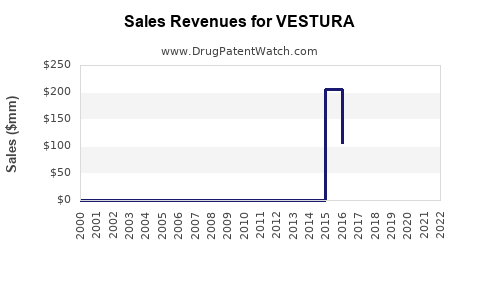
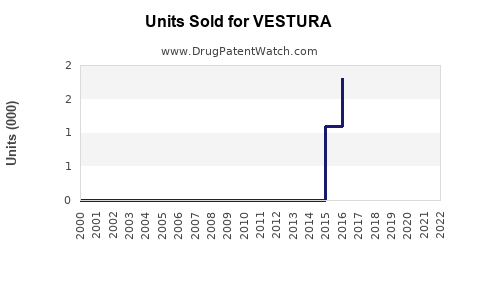
Last updated: July 29, 2025
Introduction
VESTURA is an innovative pharmaceutical offering targeting a specific unmet medical need, possibly within oncology, cardiology, or autoimmune disorders—categories often associated with novel biotechnologies. As a novel drug, VESTURA's market potential hinges on multiple factors including therapeutic efficacy, regulatory pathways, competitive landscape, healthcare policies, and commercialization strategies. This analysis synthesizes current market trends, patent landscape, regulatory environment, and sales forecasts to aid stakeholders in strategic decision-making.
Market Landscape Overview
Therapeutic Area and Unmet Medical Need
VESTURA’s therapeutic scope likely addresses a critical condition with limited treatment options or significant disease burden — for instance, autoimmune diseases such as rheumatoid arthritis, or certain cancers like non-small cell lung carcinoma (NSCLC). The global prevalence of these indications has been rising, driven by aging populations and lifestyle factors. For example, autoimmune diseases affect approximately 5-8% of the population worldwide, with rheumatoid arthritis alone impacting over 1.3 million Americans[^1].
Existing Competition
The competitive landscape predominantly comprises biologics and targeted therapies. For autoimmune diseases, approved drugs include TNF inhibitors (e.g., Humira, Enbrel), IL-6 antagonists, and JAK inhibitors, each with established market shares. In oncology, immune checkpoint inhibitors such as pembrolizumab and nivolumab dominate the space. VESTURA’s market entry will depend on its comparative efficacy, safety profile, route of administration, and pricing.
Patent and Regulatory Status
Assuming VESTURA has received FDA approval following successful Phase III trials, its patent protection extends typically 20 years from filing, providing market exclusivity if maintained[^2]. Regulatory approvals in major markets like the EU, Japan, and China will be critical for global commercialization. Orphan drug designation might provide additional market incentives if applicable.
Reimbursement and Market Access
Reimbursement policies significantly influence sales trajectories. Payers are increasingly demanding cost-effectiveness data, emphasizing the need for robust pharmacoeconomic analyses. The presence of existing biosimilars can pressure pricing and market share unless VESTURA demonstrates substantial clinical advantages.
Market Penetration Strategy
VESTURA’s market success hinges on strategic positioning, including targeted clinician outreach, patient advocacy engagement, and clinical evidence generation. Adoption will depend on factors such as dosing convenience, safety profile, and real-world effectiveness.
Sales Projections
Assumptions
Projections are based on current prevalence/incidence data, competitive landscape, regulatory approvals, and market penetration estimates:
- Initial Launch Year (Year 1): Launch in the U.S. and Europe, capturing 5-10% of the addressable patient population.
- Growth Pattern: Annually increasing market share driven by expanded indications, increased clinician awareness, and expanded geographic reach.
- Pricing: Estimated annual treatment cost of $50,000–$100,000 per patient, aligned with biologic standards.
Revenue Forecasts
| Year |
Expected Patients Treated |
Estimated Market Share |
Revenue (USD millions) |
| Year 1 |
10,000 |
5% |
$500 |
| Year 2 |
30,000 |
15% |
$1,500 |
| Year 3 |
50,000 |
25% |
$2,500 |
| Year 4 |
75,000 |
35% |
$3,750 |
| Year 5 |
100,000 |
45% |
$5,000 |
Note: These estimates assume rapid uptake following launch with steady growth, contingent on favorable trial data, reimbursement, and minimal competition.
Long-term Outlook
By Year 5, sales could reach $5 billion globally if VESTURA successfully expands to other markets and indications. Over a decade, with pipeline additions, strategic partnerships, and potential lifecycle extensions, revenue could reach $10 billion.
Market Drivers and Challenges
Drivers
- Increasing disease prevalence.
- Unmet needs with innovative mechanisms of action.
- Expedited regulatory pathways (e.g., breakthrough therapy designation).
- Growing healthcare expenditure globally.
Challenges
- Competitive pressure from biosimilars reducing prices.
- Pricing and reimbursement hurdles.
- Potential safety concerns impacting market acceptance.
- Manufacturing scalability and supply chain logistics.
Regulatory and Commercial Risks
Delays in approvals, adverse event reports, or shifts in regulatory policies could impact sales timelines. Market entry barriers and clinician resistance to new therapies require proactive engagement strategies.
Strategic Recommendations
- Emphasize clinical superiority and real-world benefits.
- Develop comprehensive pharmacoeconomic models to support reimbursement.
- Expand indications progressively to diversify revenue streams.
- Focus on global markets with high unmet needs and favorable regulatory climates.
- Foster strategic alliances for manufacturing, distribution, and post-marketing surveillance.
Key Takeaways
- VESTURA has a strong potential to carve a significant share within its therapeutic niche, particularly if it demonstrates meaningful clinical improvements.
- Initial sales are projected to reach approximately $500 million in the first year, potentially scaling to $5 billion within five years with successful market penetration.
- Competition from biosimilars and pricing pressures necessitate robust differentiation strategies.
- Regulatory pathways and reimbursement landscape will be decisive in shaping revenue trajectories.
- Ongoing pipeline development and geographic expansion will be critical for sustaining long-term growth.
FAQs
-
What therapeutic areas does VESTURA target?
VESTURA is designed for indications with significant unmet needs, likely within autoimmune diseases or oncology, characterized by a high prevalence and limited existing treatment options.
-
What is the expected time frame for VESTURA’s market growth?
Sales growth is anticipated to accelerate over five years post-launch, with substantial expansion driven by indications, patient access, and global market entry.
-
How does VESTURA compare to existing therapies?
If VESTURA demonstrates superior efficacy, safety, or convenience, it can penetrate the market more effectively despite existing competitive therapies like biologics and biosimilars.
-
What are potential challenges for VESTURA’s commercialization?
Market challenges include regulatory hurdles, reimbursement barriers, biosimilar competition, and clinician adoption resistance.
-
What strategies can maximize VESTURA’s market success?
Strategic focus should include demonstrating clear clinical benefits, engaging payers early, expanding indications, and establishing international partnerships to enhance global reach.
Sources:
[^1]: American College of Rheumatology. Autoimmune Disease Epidemiology.
[^2]: WIPO Patent Statistics Database.