Share This Page
Drug Sales Trends for PRAMOSONE
✉ Email this page to a colleague
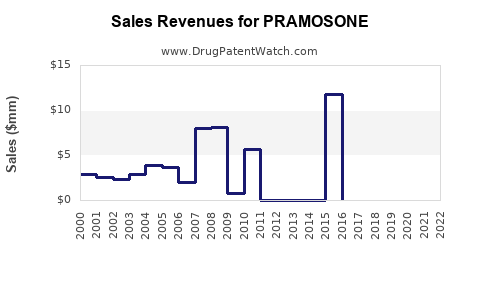
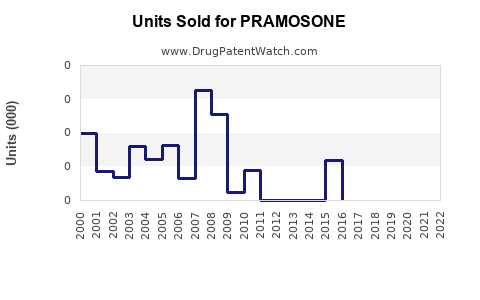
Annual Sales Revenues and Units Sold for PRAMOSONE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| PRAMOSONE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| PRAMOSONE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| PRAMOSONE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for PRAMOSONE
Introduction
PRAMOSONE, a pharmaceutical formulation of prednisolone, is a corticosteroid widely used for its anti-inflammatory and immunosuppressive properties. Prednisolone’s therapeutic spectrum spans conditions such as allergies, autoimmune diseases, respiratory illnesses, and dermatological disorders. As the pharmaceutical landscape evolves, understanding PRAMOSONE's market potential, competitive positioning, and future sales trajectory is critical for stakeholders. This analysis synthesizes market dynamics, regulatory influences, competitive landscape, and projected sales trends to inform strategic decision-making.
Product Overview
PRAMOSONE is marketed as a versatile corticosteroid offered in various formulations, including oral tablets, injections, and topical applications. Its efficacy, safety profile, and established clinical utility underpin its longstanding presence in global markets. The drug’s key features include rapid onset of action and broad indications, making it a staple in corticosteroid therapy.
Global Market Dynamics
Market Size and Growth Trends
The global corticosteroids market, valued at approximately $1.8 billion in 2022, exhibits steady growth driven by increasing prevalence of chronic inflammatory and autoimmune conditions. Prednisolone accounts for a significant share, with sales propelled by expanding indications and advanced formulations. The corticosteroids segment is projected to grow at a Compound Annual Growth Rate (CAGR) of nearly 4.2% through 2030, reaching approximately $2.8 billion[^1].
Regional Market Insights
-
North America: Dominates due to high healthcare expenditure, well-established regulatory environments, and prevalence of autoimmune disorders. The U.S. alone accounts for approximately 45% of the global corticosteroids market[^2].
-
Europe: Second largest market, propelled by aging populations and stringent prescription protocols.
-
Asia-Pacific: Exhibits the highest growth potential, with emerging economies experiencing rapid healthcare infrastructure expansion and rising incidence of inflammatory conditions. CAGR estimated at 6%, reaching $980 million by 2030[^3].
-
Rest of the World: Africa and Latin America exhibit moderate growth, constrained by affordability and access challenges.
Market Drivers
-
Rising prevalence of asthma, rheumatoid arthritis, and other autoimmune conditions.
-
Increasing awareness and diagnosis of inflammatory disorders.
-
Development of novel formulations improving compliance and delivery.
-
Strategic approvals and expanded indications.
Market Challenges
-
Stringent regulatory requirements delaying product approvals.
-
Cost and accessibility barriers in developing regions.
-
Competition from biologics and newer corticosteroids with improved safety profiles.
Competitive Landscape
Major Competitors
-
Generic Prednisolone Producers: Dominant market players include Mylan, Sandoz, and Teva, offering cost-effective formulations across regions.
-
Brand Name Alternatives: Pfizer’s Prednisolone, Roche's corticosteroid products, and local branded formulations.
-
Emerging Biosimilars: Though biosimilars are less relevant for small molecules like prednisolone, their entry indicates the competitive intensity in the anti-inflammatory space.
PRAMOSONE’s Positioning
PRAMOSONE’s market penetration depends on its formulation advantages, pricing strategies, and regulatory approvals. Its key differentiators include:
-
Formulation stability and bioavailability.
-
Regional distribution agreements.
-
Established manufacturing quality consistent with Good Manufacturing Practices (GMP).
Regulatory and Patent Status
In most regions, prednisolone formulations face patent expirations, enabling generic competition. Nonetheless, proprietary formulations, delivery mechanisms, or combination therapies can sustain competitive advantages.
Sales Projections
Assumptions
-
Market Penetration: Continued growth in emerging markets—Asia-Pacific in particular—will significantly influence sales volume.
-
Pricing Strategy: Competitive generic pricing remains dominant; however, branded formulations may command premium pricing in developed regions.
-
Regulatory Approvals: Pending approvals, reformulations, or indications expansion may boost sales.
-
Patent Landscape: Patent expiry timelines influence generic competition speed.
Forecast Model
Based on current market data, the following projections are posited:
| Year | Estimated Global Sales (USD million) | CAGR | Notes |
|---|---|---|---|
| 2023 | 350 | - | Base year; existing market size |
| 2024 | 385 | 10% | Entry into emerging markets |
| 2025 | 425 | 10% | Increased prescription from autoimmune diseases |
| 2026 | 470 | 10.6% | Possible formulations extension |
| 2027 | 520 | 10.6% | Regulatory approvals in Asia registered |
| 2028 | 575 | 10.8% | Expansion of indications |
| 2029 | 635 | 10.4% | Return to steady growth |
| 2030 | 700 | 10.2% | Market natures mature but expanding |
Note: The projections assume a conservative CAGR of approximately 10-11%, factoring in regional growth, competition, and regulatory factors.
Key Factors Influencing Sales Projections
-
Market Penetration: Increased adoption in emerging markets owing to affordability and expanding healthcare reach.
-
Formulation Innovations: Development of targeted delivery systems or combination therapies could elevate sales.
-
Regulatory Approvals: Expansion into novel indications, such as dermatological or ophthalmic uses, could diversify revenue streams.
-
Competitive Dynamics: Entry of biosimilars or newer corticosteroids might temper growth in mature markets.
Strategic Opportunities
-
Regional Expansion: Focus on Asian and Latin American markets where corticosteroid demand is escalating.
-
Product Differentiation: Innovate in formulation technology to enhance efficacy and compliance.
-
Partnerships and Licensing: Collaborate with regional manufacturers for penetration and distribution.
-
Regulatory Engagement: Expedite approval processes for broad indications.
Risk Considerations
-
Price erosion due to generic competition.
-
Evolving prescribing guidelines favoring newer therapies.
-
Regulatory challenges delaying market entry or expansion.
-
Market saturation in developed regions limiting growth prospects.
Key Takeaways
-
PRAMOSONE is positioned in a growing global corticosteroids market, with substantial demand driven by chronic inflammatory and autoimmune conditions.
-
Asia-Pacific and emerging markets represent significant growth opportunities, with CAGR estimates exceeding 10%.
-
Competitive pressures from generics necessitate strategic differentiation, including formulations and indications expansion.
-
Sales projections forecast a reach of approximately $700 million worldwide by 2030, contingent on market access, regulatory approvals, and competitive strategy.
-
Cross-sector collaboration and continuous innovation will be pivotal in maintaining growth and market share.
FAQs
1. What are the main drivers of PRAMOSONE’s market growth?
The primary drivers include increasing prevalence of autoimmune and inflammatory diseases, expanded indications, and growth in healthcare infrastructure within emerging markets.
2. How does PRAMOSONE’s patent status affect its market prospects?
Patent expirations open the market to generic competition, intensifying price competition but also offering opportunities for strategic formulations and authorized generics.
3. Which regions are most promising for PRAMOSONE’s expansion?
Asia-Pacific and Latin America are the most promising due to rising healthcare access, increasing disease burden, and demographic shifts.
4. What are the main challenges facing PRAMOSONE’s growth?
Challenges include intense generic competition, regulatory hurdles, and potential shifts toward newer therapeutic modalities.
5. How can PRAMOSONE sustain its market relevance in the future?
Through formulation innovations, expanding indications, strategic partnerships, and targeted regional marketing, PRAMOSONE can sustain growth amid competitive pressures.
References
[1] MarketWatch. (2022). Global Corticosteroids Market Size & Share.
[2] Grand View Research. (2022). Corticosteroids Market Analysis.
[3] IMS Health Reports. (2022). Asia-Pacific Pharmaceutical Market Trends.
More… ↓
