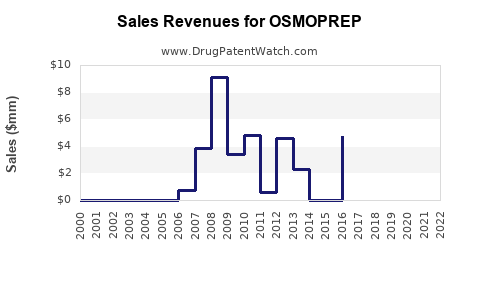
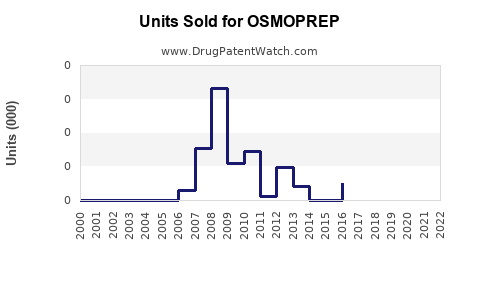
Last updated: July 27, 2025
Introduction
OSMOPREP (sodium picosulfate, magnesium oxide, and citric acid) is an oral bowel preparation drug marketed as a low-volume, patient-friendly solution for colon cleansing prior to colonoscopy. Its formulation aims to improve patient compliance and bowel cleansing efficacy compared to traditional high-volume solutions like polyethylene glycol (PEG). Analyzing its market landscape and projecting sales trajectories involves assessing current demand drivers, competitive positioning, regulatory factors, and broader healthcare trends.
Market Overview
The global bowel preparation agents market was valued at approximately USD 900 million in 2022, with a compound annual growth rate (CAGR) of about 5%, projected to reach USD 1.2 billion by 2028 (Source: MarketsandMarkets). The increasing prevalence of colorectal cancer, rising screening programs, and technological advancements in endoscopy procedures underpin this growth. The market is characterized by a mix of generic and branded drugs, with a notable shift towards patient-friendly formulations to boost compliance.
Key Market Drivers
-
Rising Colorectal Cancer Incidence: Globally, colorectal cancer ranks among the leading causes of cancer-related mortality. Early detection through colonoscopy increases demand for effective bowel prep solutions. The WHO estimates approximately 1.9 million new cases diagnosed in 2020 alone, fueling screening initiatives ([1]).
-
Enhanced Patient Compliance: Traditionally, high-volume PEG solutions posed tolerability issues. OSMOPREP’s low-volume formulation (5.5 ounces vs. 45-quote ounces of PEG) significantly improves palatability, compliance, and overall patient satisfaction, thus boosting demand.
-
Growing Screening Programs: Countries with expanding healthcare coverage and established screening protocols, especially in North America and Europe, reinforce the need for reliable bowel prep drugs.
-
Technological Advancements: Innovations in bowel cleansing formulations—such as flavored, low-volume, and split-dose regimens—favor products like OSMOPREP.
Competitive Landscape
OSMOPREP operates within a competitive environment featuring several established brands:
-
Mirror Paxlovid and MoviPrep: Traditionally dominant, these brands emphasize high-volume solutions and varied dosing schedules.
-
Pharmacoeconomic Factors: Reimbursement policies influence drug selection, with insurers favoring cost-effective options.
-
Patient Preference: The low-volume nature of OSMOPREP is a strategic advantage against bulkier alternatives, enhancing prescription rates.
Manufacturers must navigate stringent regulatory standards and demonstrate clinical efficacy and safety to capture increased use.
Regulatory and Reimbursement Dynamics
OSMOPREP’s approval by agencies such as the FDA in the U.S. and the EMA in Europe establishes credibility. Reimbursement policies impact market penetration; coverage under government and private insurance plans facilitates broader access. Recent regulatory emphasis on patient safety and tolerability metrics aligns with OSMOPREP’s attributes, fostering favorable reimbursement strategies.
Sales Projections
Short-term (1-2 years):
-
Market Adoption: Initial uptake driven by targeted marketing to gastroenterologists and endoscopy centers. Early adopter clinics, emphasizing patient comfort, are likely to prescribe OSMOPREP as a preferred bowel prep.
-
Projected Sales: Estimated at USD 50-75 million annually within North America and Europe, considering current market share and demand growth. Early adoption rates suggest a compound growth rate of approximately 10-15%.
Medium-term (3-5 years):
-
Market Penetration: As awareness increases, broader adoption across hospital and outpatient settings can expand usage. Enhanced competition may moderate growth but leveraging value propositions—such as patient adherence—can sustain momentum.
-
Projected Sales: Reaching USD 150-200 million globally, supported by expanding geographical footprint into Asia-Pacific and Latin America, where colorectal screening initiatives expand.
Long-term (5+ years):
-
Market Saturation and Innovation: Adoption may plateau as newer formulations or delivery systems emerge. Strategic differentiation and expansion into complementary indications—such as bowel management for other gastrointestinal procedures—may augment sales.
-
Projected Sales: Potential to surpass USD 250 million annually if integrated with comprehensive colorectal cancer screening programs and evolving endoscopic techniques.
Challenges and Opportunities
Challenges:
-
Market Competition: Entrenched brands and generics may impede rapid growth unless OSMOPREP demonstrates superior clinical outcomes or cost advantages.
-
Regulatory Hurdles: Differing approvals and evolving safety standards could delay market expansion.
-
Patient Preferences: Despite low-volume advantages, taste, ease of administration, and adverse events influence prescription patterns.
Opportunities:
-
Expanding Indications: Potential use in bowel cleansing for other diagnostic or surgical procedures.
-
Digital Health Integration: Promoting patient education and adherence through digital platforms enhances effectiveness.
-
Partnerships: Collaborations with healthcare providers and payers to optimize formulary placement.
Regional Market Trends
North America: Leading market due to high colorectal cancer screening rates (~68% compliance in the U.S.), well-established healthcare infrastructure, and favorable reimbursement. Growth influenced by increasing awareness and preference for patient-friendly solutions.
Europe: Growing adoption, reinforced by national screening programs, with significant cost-containment efforts encouraging use of effective, low-volume agents.
Asia-Pacific and Latin America: Emerging markets showing robust growth due to increasing healthcare investments, rising awareness, and expanding screening initiatives.
Key Factors Influencing Sales Growth
-
Demographic Shifts: Aging populations globally increase the need for screening, expanding potential market.
-
Clinical Guidelines: Adoption of guidelines emphasizing low-volume bowel preparations enhances prescription rates.
-
Competitive Pricing Strategies: Price optimization aligned with regional economic conditions influences formulary inclusion.
-
Real-World Evidence: Demonstrations of safety, efficacy, and improved patient compliance promote clinician prescribing patterns.
Conclusion
OSMOPREP is poised to benefit from the expanding demand for patient-centric bowel preparations driven by rising colorectal cancer screening and technological innovation. Short-term sales are expected to grow steadily, with significant potential in North America and Europe. Medium to long-term projections hinge on market penetration, competition management, and adoption into new indications. Strategic positioning emphasizing its tolerability and efficacy will be critical to capturing market share in a competitive landscape.
Key Takeaways
-
The global bowel prep market is expanding, driven by increased colorectal cancer screening and technological advancements, positioning OSMOPREP favorably.
-
Its low-volume, patient-friendly formulation offers a competitive edge over traditional solutions, facilitating higher compliance.
-
Short-term sales estimates range between USD 50-75 million annually, with potential to reach USD 150-200 million within 3-5 years.
-
Growth depends on regulatory acceptance, reimbursement policies, and effective marketing to gastroenterologists and healthcare systems.
-
Opportunities include expanding indications, leveraging digital health tools, and forming strategic partnerships to sustain growth.
FAQs
1. How does OSMOPREP compare with traditional bowel preparations like PEG solutions?
OSMOPREP offers a low-volume, flavored formulation resulting in better patient compliance and tolerability compared to high-volume PEG solutions, which often cause discomfort and poor adherence.
2. What are the primary drivers for OSMOPREP’s market growth?
Key drivers include rising colorectal cancer screening rates, patient preference for less burdensome preparations, and technological advancements in bowel cleansing.
3. What regions present the most promising growth opportunities for OSMOPREP?
North America and Europe currently dominate, but expanding into Asia-Pacific and Latin America offers substantial future potential due to increasing healthcare investments and screening programs.
4. What challenges could impede OSMOPREP’s sales growth?
Intense competition from established brands, regulatory hurdles, and clinician inertia towards switching formulations are notable challenges.
5. How will reimbursement policies influence OSMOPREP’s market penetration?
Positive reimbursement frameworks and formulary placements will significantly facilitate broader adoption, whereas restrictive policies may limit sales growth.
References
- World Health Organization. Colorectal Cancer Fact Sheet. 2021.