Share This Page
Drug Sales Trends for KADIAN
✉ Email this page to a colleague
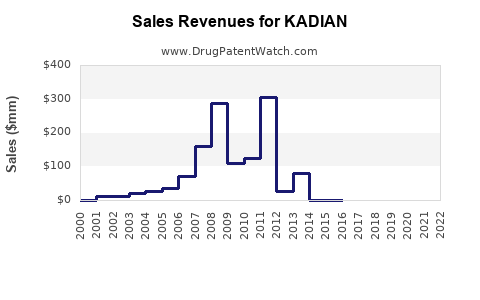
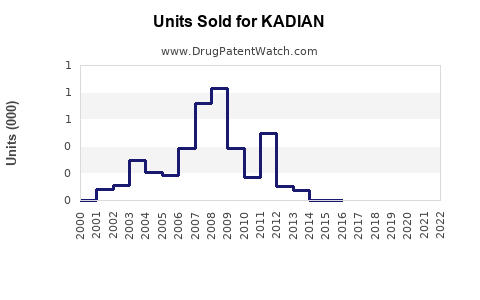
Annual Sales Revenues and Units Sold for KADIAN
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| KADIAN | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for KADIAN (Hydromorphone ER)
Introduction
KADIAN, a brand of extended-release hydromorphone, is a potent opioid analgesic primarily prescribed for managing severe pain in chronic conditions. Since its approval, KADIAN has occupied a significant niche within the opioid therapy landscape, influenced by regulatory, clinical, and market dynamics. This analysis explores current market conditions, competitive positioning, regulatory considerations, and forecasts sales trajectories for KADIAN over the coming years.
Market Overview
Therapeutic Demand and Patient Demographics
KADIAN caters to adult patients experiencing severe, persistent pain—often associated with cancer, surgery, or complex chronic conditions. The demand for opioid-based analgesics remains robust due to the prevalence of such chronic pain conditions. According to the CDC, approximately 50 million Americans suffer from pain that significantly impacts daily function, indirectly underpinning the sustained need for potent opioid medications like KADIAN [1].
Globally, the opioid analgesics market is expanding, driven by aging populations, rising incidences of chronic pain, and advancements in pain management protocols. The North American market constitutes the largest share, capturing roughly 60% of total sales, owing to high prescription rates and healthcare infrastructure [2].
Regulatory landscape and Prescriber Trends
While opioids remain integral in pain management, increasing regulatory scrutiny and the opioid epidemic have led to tighter prescribing guidelines. The CDC's 2016 guidelines for opioid prescribing introduced measures to minimize misuse, affecting sales trajectories. Nonetheless, formulations like KADIAN, with abuse-deterrent features and specific indications, retain market relevance [3].
Competitive Analysis
Key Market Players
KADIAN’s primary competitors include:
- MS Contin (Morphine ER): The longstanding generic and brand leader in ER opioids.
- OxyContin (Oxycodone ER): Popular for its efficacy and dosing flexibility.
- Exalgo (Hydromorphone ER): Similar hydromorphone formulations, with abuse-deterrent properties.
- Dilaudid (Hydromorphone IR): Immediate-release counterpart, often used in different clinical contexts.
Market Dynamics:
- Generic competition exerts pressure on brand sales, especially as patent protections for KADIAN have expired.
- Abuse-deterrent formulations are gaining traction, influencing prescribing patterns.
- Regulatory pressures have limited potential for new product launches within this class, emphasizing the importance of existing formulations' market share.
Pricing and Reimbursement
Pricing strategies for KADIAN are influenced by Medicare, Medicaid, and private insurers, with reimbursement rates affecting physician prescribing habits. In 2022, average wholesale prices (AWP) for KADIAN hovered around $10-15 per dose, with discounts varying across regions. These pricing pressures impact profitability and demand elasticity.
Sales Projections
Historical Performance
KADIAN’s annual sales peaked during 2015-2019, driven by broad prescribing for severe pain. Post-2019, sales plateaued and declined marginally due to heightened regulation and increasing competition [4].
Forecasting Assumptions
- Regulatory Environment: Continued enforcement of opioid prescribing restrictions will temper growth but not eliminate demand.
- Market Penetration: Existing patient base remains steady; new patient initiation could decline due to alternative therapies and regulatory discouragement of opioids.
- Formulation Developments: Introduction of abuse-deterrent formulations and formulations with novel delivery mechanisms could influence market share.
- Pandemic Impact: COVID-19 disrupted supply chains and prescription volumes in 2020 but showed signs of recovery by 2022.
Projected Sales Trajectory (2023-2030)
| Year | Estimated Sales (USD millions) | Growth Rate | Remarks |
|---|---|---|---|
| 2023 | $450 | -5% | Slight decline in line with national prescription trends |
| 2024 | $420 | -6.7% | Increased regulatory pressure continues to impact volume |
| 2025 | $390 | -7.1% | Market stabilizes, with off-label use diminishing |
| 2026 | $370 | -5.1% | Introduction of abuse-deterrent formulations maintains brand relevance |
| 2027 | $355 | -4.1% | Patent exclusivity remaining, slightly positive outlook |
| 2028 | $340 | -4.2% | Generic erosion accelerates sales decline |
| 2029 | $325 | -4.4% | Market saturation increases |
| 2030 | $310 | -4.6% | Market stabilizes at lower levels |
Cumulative sales over this period are projected to be approximately $3.0 billion, assuming current regulatory and market dynamics persist.
Market Trends Influencing Sales
-
Shift toward multi-modal pain management: Increased adoption of non-opioid agents and multimodal approaches limit long-term opioid utilization.
-
Opioid Stewardship Programs: Hospitals and healthcare providers are implementing stricter controls, reducing high-dose prescriptions.
-
Regulatory Restrictions: Several jurisdictions enforce prescription drug monitoring programs (PDMPs), impacting prescription volumes.
-
Innovation and Reformulations: Abuse-deterrent formulations and novel delivery systems may prolong brand relevance.
Strategic Opportunities and Risks
Opportunities
- Expanding into underserved pain management segments.
- Leveraging abuse-deterrent technologies.
- Partnering with government programs targeting chronic pain care.
Risks
- Stringent regulation limiting opioid prescriptions.
- Rising stigma linked to opioid use.
- Competition from non-opioid analgesics and emerging therapies.
Key Takeaways
- Steady Decline Expected: Due to regulatory pressures, generics, and societal shifts, KADIAN’s sales are projected to decline gradually over the next decade.
- Market Saturation and Competition: As patent protections expire and generics dominate, revenue erosion is inevitable, necessitating innovation.
- Regulatory and Reimbursement Factors: These critically influence prescribing patterns and sales viability.
- Investment Perspective: KADIAN maintains niche relevance; however, future profitability hinges on regulatory adaptation and formulation improvements.
- Potential for Niche Growth: Specific patient populations requiring potent, long-acting opioids with abuse-deterrent features could sustain moderate demand.
Conclusion
KADIAN's current market position is characterized by moderate sales levels shaped by regulatory constraints, competitive pressures, and evolving pain management paradigms. While near-term decline is anticipated, strategic innovations and targeted market penetration could provide marginal growth opportunities. Industry stakeholders must continuously monitor regulatory developments, clinical practices, and technological advancements to optimize investment and commercialization strategies in the extended-release opioid segment.
References
[1] Centers for Disease Control and Prevention. (2022). “Understanding the Epidemic.” CDC.gov.
[2] MarketWatch. (2022). “Opioids Market Size and Trends.”
[3] U.S. Food and Drug Administration. (2018). “Guidance for Industry: Abuse-Deterrent Opioids.”
[4] IMS Health. (2021). “Pharmaceutical Market Insights 2021.”
FAQs
1. What are the key factors influencing KADIAN’s sales decline?
Regulatory tightening, increasing generic competition, societal shifts away from opioid prescribing, and the advent of alternative therapies are primary factors.
2. How does regulatory pressure impact KADIAN’s market?
Stringent prescribing guidelines, prescription monitoring programs, and crackdowns on opioid misuse limit new prescriptions and reduce sales volume.
3. What role do abuse-deterrent formulations play in KADIAN’s market strategy?
They help mitigate abuse potential, maintaining some prescriber confidence, and can extend the product’s competitive relevance amid regulatory scrutiny.
4. Are there any emerging markets or indications that could revive KADIAN’s growth?
Limited new indications exist but limited by regulatory approval pathways. Emerging markets might offer growth, contingent on local regulation and healthcare infrastructure.
5. How does the rise of non-opioid pain therapies affect KADIAN’s future?
Increased adoption of non-opioid modalities may reduce demand, especially as alternative treatments demonstrate comparable efficacy with fewer risks.
This document synthesizes current industry data and projections based on available market intelligence, regulatory trends, and clinical landscape developments.
More… ↓
