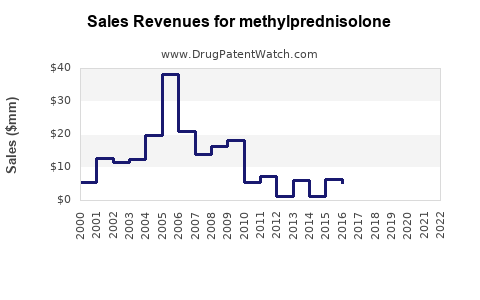
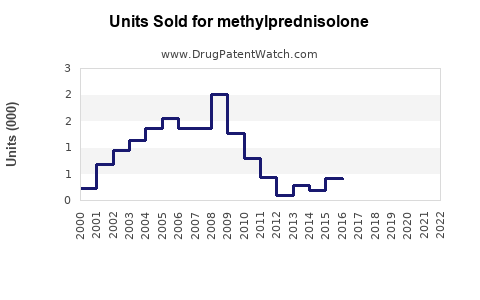
Last updated: July 29, 2025
Introduction
Methylprednisolone, a synthetic corticosteroid with potent anti-inflammatory and immunosuppressive properties, occupies a significant position in the pharmaceutical market. It is broadly utilized for treating inflammatory conditions, autoimmune disorders, allergic reactions, and certain types of cancer. Given its established therapeutic profile and widespread clinical applications, understanding the market landscape and projecting future sales are vital for stakeholders including pharmaceutical companies, investors, and healthcare policy-makers. This analysis provides a comprehensive review of the current market environment, key growth drivers, competitive landscape, and sales forecasts for methylprednisolone over the next five years.
Market Overview
Global Market Size
The global corticosteroids market, which includes methylprednisolone, was valued at approximately USD 2.2 billion in 2022, with methylprednisolone representing a significant share owing to its extensive clinical usage. According to industry reports [1], the corticosteroids segment is projected to grow at a compound annual growth rate (CAGR) of around 4% through 2028, driven primarily by increasing prevalence of autoimmune and inflammatory diseases.
Therapeutic Indications and Usage Patterns
Methylprednisolone is primarily indicated for conditions such as multiple sclerosis relapses, severe allergic reactions, skin diseases, arthritis, and respiratory disorders. Its parenteral formulation, including intravenous and intramuscular injections, is favored for acute conditions, while oral forms are used for chronic management.
Market Dynamics
The demand for methylprednisolone is influenced by factors such as rising prevalence of autoimmune diseases (e.g., multiple sclerosis, rheumatoid arthritis), expanding indications, and increasing adoption in inpatient and outpatient settings. Additionally, the COVID-19 pandemic underscored the importance of corticosteroids like methylprednisolone in managing cytokine storms, further boosting its usage [2].
Key Market Drivers
-
Rising Incidence of Autoimmune and Inflammatory Disorders:
The global burden of autoimmune diseases is increasing, notably in developed economies, prompting higher corticosteroid utilization. The WHO reports an estimated 80 autoimmune diseases affecting hundreds of millions worldwide [3].
-
Expansion of Indications:
Emerging evidence supports methylprednisolone in novel therapeutic areas, including COVID-19 management, which adds to its market appeal. The NIH’s widespread recommendation of corticosteroids for severe COVID-19 cases significantly amplified demand [4].
-
Innovation and Formulation Developments:
Development of high-bioavailability formulations and outpatient administration options is improving patient adherence and expanding clinical application scope.
-
Growing Healthcare Expenditure:
Increased healthcare spending, particularly in emerging economies, facilitates access to corticosteroids, including methylprednisolone.
Competitive Landscape
The market comprises several key players, including Pfizer, Sandoz (a division of Novartis), Teva Pharmaceuticals, Mylan (now part of Viatris), and local generic manufacturers. Pfizer's methylprednisolone acetate presents a prominent branded product, while generics dominate due to cost advantages. Patent expirations and regulatory approvals of generics have intensified price competition and broadened market accessibility.
Regulatory Environment:
Adherence to stringent regulations by agencies like the FDA, EMA, and local authorities influences market dynamics. While methylprednisolone's patents have largely expired, ongoing approvals for new formulations and indications provide competitive advantages.
Regional Market Insights
-
North America:
Leading in market share, driven by high disease prevalence, advanced healthcare infrastructure, and increasing approval of new indications. The U.S. accounted for approximately 45% of the global methylprednisolone sales in 2022.
-
Europe:
The second-largest market, with steady growth, fueled by aging populations and regulatory approvals across member states.
-
Asia-Pacific:
Expected to exhibit the highest CAGR (~6%) owing to expanding healthcare access, rising autoimmune disease cases, and population growth. Countries like China and India represent burgeoning markets.
Challenges and Market Barriers
-
Side Effect Profile and Long-term Usage Concerns:
Adverse effects such as osteoporosis, hyperglycemia, and immunosuppression limit long-term application, potentially restraining market growth.
-
Pricing and Patent Dynamics:
Generic competition and pricing pressures impact profit margins. Regulatory hurdles and variable approval timelines also pose challenges.
-
Emergence of Biologic Alternatives:
Growing adoption of biologic agents for autoimmune conditions may reduce corticosteroid dependence over time.
Sales Projections (2023–2028)
Based on current trends, the following projections are outlined:
| Year |
Estimated Global Sales (USD Billion) |
CAGR (%) |
| 2023 |
2.5 |
— |
| 2024 |
2.63 |
5% |
| 2025 |
2.76 |
5% |
| 2026 |
2.89 |
5% |
| 2027 |
3.03 |
5% |
| 2028 |
3.18 |
5% |
The conservative annual growth rate of 5% reflects factors including continued demand in existing indications, expansion into COVID-19 related treatment protocols, and the growth of Asian markets. This forecasts an industry worth approximately USD 3.18 billion by 2028, with sustained demand driven by global disease burden and healthcare investments.
Strategic Opportunities
-
Formulation Enhancements:
Developing novel delivery systems, such as sustained-release or inhalation formulations, could capture underserved niches.
-
Expanding Indications:
Further clinical research can open new therapeutic domains for methylprednisolone, including dermatological and neurological conditions.
-
Emerging Markets Penetration:
Focused marketing and regulatory strategies in Asia-Pacific and Latin America can accelerate sales growth.
-
Partnership and Licensing:
Collaborations with local manufacturers can facilitate market entry and expand access.
Key Takeaways
-
Robust Market Profile: Methylprednisolone maintains a vital role within the corticosteroid market, supported by high prevalence of autoimmune and inflammatory diseases and expanding indications.
-
Growth Trajectory: Projected to grow at approximately 5% annually through 2028, reaching over USD 3 billion globally.
-
Regional Expansion: North America remains dominant; however, Asia-Pacific is poised for significant growth, presenting strategic opportunities.
-
Competitive Landscape: Dominated by generic manufacturers post-patent expiry; innovation and formulation improvements offer competitive advantages.
-
Challenges and Risks: Adverse effect concerns, regulatory hurdles, and competition from biologics require ongoing strategic adaptations.
Conclusion
Methylprednisolone’s entrenched clinical utility, combined with expanding therapeutic applications and global healthcare investments, underpin a stable growth trajectory over the next five years. Manufacturers that innovate in formulations, expand into emerging markets, and pursue new indications will be well-positioned to capitalize on this market potential.
FAQs
1. What are the primary indications driving methylprednisolone sales?
Autoimmune disorders (e.g., multiple sclerosis, rheumatoid arthritis), allergic reactions, inflammatory skin conditions, and acute respiratory issues are primary drivers, with additional demand from COVID-19 management protocols.
2. How has patent expiry affected the methylprednisolone market?
Patent expiry has led to an influx of generic products, intensifying price competition and expanding access, especially in developing regions. It has also prompted manufacturers to innovate formulations and new indications to maintain market share.
3. What are the main challenges facing methylprednisolone manufacturers?
Adverse effects limiting long-term use, regulatory complexities, competition from biologics, and pricing pressures are key challenges impacting profitability and market growth.
4. Which regions offer the most growth potential for methylprednisolone?
While North America remains the largest market, Asia-Pacific offers the highest growth potential due to demographic shifts, increased healthcare access, and rising disease prevalence.
5. What opportunities exist for future growth?
Developing novel formulations, expanding indication spectrum, penetrating emerging markets, and engaging in strategic partnerships are strategic avenues to boost sales and market share.
References
[1] MarketWatch, "Global Corticosteroids Market Size, Share & Trends Analysis Report," 2022.
[2] Johns Hopkins University, "Steroids in COVID-19 Treatment," 2021.
[3] WHO, "Autoimmune Diseases Fact Sheet," 2020.
[4] NIH, "COVID-19 Treatment Guidelines," 2022.