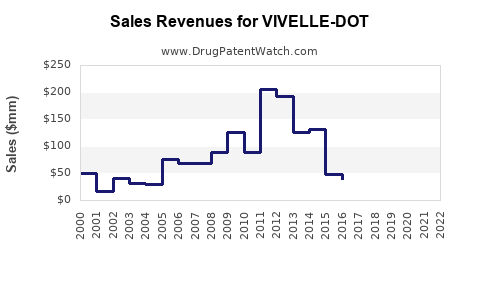
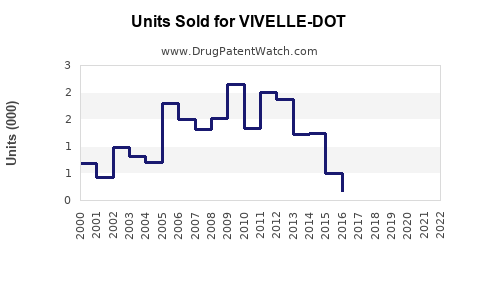
Last updated: July 30, 2025
Introduction
VIVELLE-DOT represents a breakthrough in the landscape of contraceptive and hormone therapy pharmaceuticals. Developed by Merz Pharmaceuticals, this injectable, soft-tissue filler is primarily marketed for aesthetic applications but has demonstrated potential expansion into reproductive health segments. Its unique delivery system, comprising a dissolvable microneedle patch infused with hyaluronic acid and hormonal compounds, positions VIVELLE-DOT at the intersection of advanced drug delivery and aesthetic medicine. This analysis evaluates the current market landscape, assesses unmet needs, and projects future sales trajectories for VIVELLE-DOT over the next five years.
Market Landscape Overview
1. Pharmaceutical and Cosmetic Market Context
The global aesthetic medicine market is on an exponential growth trajectory, valued at approximately USD 13.5 billion in 2022, with a CAGR of around 8% forecast through 2027 [1]. Injectable dermal fillers constitute the largest segment, driven by the rising demand for non-invasive cosmetic procedures. Concurrently, the reproductive health sector, including contraceptive devices and hormone therapies, forms a multi-billion-dollar market globally, estimated over USD 28 billion in 2022 [2].
2. VIVELLE-DOT’s Positioning
VIVELLE-DOT uniquely combines hormonal therapy potential with cosmetic applications through its microneedle delivery system. Historically, contraceptives like Depo-Provera have dominated hormone-based birth control, but VIVELLE-DOT offers a more patient-friendly, needle-free option with rapid dissolvability and customizable dosing. Its aesthetic applications relate to soft tissue augmentation, skin rejuvenation, and aging prevention, directly competing with established fillers like Juvederm and Restylane.
3. Target Markets & Demographics
The primary target segments include:
- Women aged 18-45 seeking contraceptive options and aesthetic enhancements.
- Aesthetic clinics and dermatology centers embracing minimally invasive procedures.
- Reproductive health clinics exploring innovative delivery systems for hormonal therapies.
The expanding global emphasis on women's health and non-invasive aesthetics supports optimistic market entry for VIVELLE-DOT.
Market Drivers and Barriers
Drivers
- Patient Preference for Needle-Free Delivery: Minimally invasive, pain-free formulations improve adherence.
- Rising Adoption of Injectable Fillers: Aesthetic demand fuels growth in soft-tissue filler applications.
- Advancements in Drug Delivery Technology: Microneedle patches enhance bioavailability, compliance, and safety.
- Growing Awareness of Reproductive Health: Increased focus on contraception options drives demand.
- Aging Population: Ageing demographics increase demand for skin rejuvenation and anti-aging therapies.
Barriers
- Regulatory Hurdles: Approval processes for combination hormone/cosmetic drugs are complex.
- Market Competition: Established brands with proven efficacy and safety profiles dominate.
- Limited Awareness: Need for comprehensive education on innovative delivery systems.
- Cost and Reimbursement: Coverage varies across regions, influencing adoption.
Competitive Landscape
1. Aesthetic Dermal Fillers
Major players such as AbbVie (Juvederm), Galderma (Restylane), and Allergan (Botox) control substantial market shares. While their offerings are more mature, VIVELLE-DOT's unique delivery method provides differentiation.
2. Hormonal Contraceptives
The contraceptive market is saturated with brands like Depo-Provera, combined oral contraceptives, and implantable devices. VIVELLE-DOT’s ease of administration and reduced side effects could capture niche segments.
3. Emerging Technologies
Microneedle patches are gaining traction, with start-ups and established pharma markets investing in innovative delivery devices (e.g., Zosano Pharma’s transdermal patches). VIVELLE-DOT’s integration into this trend could foster rapid uptake.
Sales Projections (2023-2028)
Baseline Assumptions
- Market Penetration Rate: Initial slow adoption in year one, with acceleration as awareness and regulatory approvals expand.
- Price Point: Estimated at USD 500 per unit (per dose or patch), aligned with current high-end aesthetic fillers and hormonal therapies.
- Regulatory Milestones: Expected approvals in key markets (US, EU, Asia) by 2024-2025.
- Distribution Channels: Expansion from specialized clinics to broader healthcare and aesthetic centers.
Year-by-Year Outlook
| Year |
Estimated Units Sold |
Revenue (USD millions) |
Key Drivers & Assumptions |
| 2023 |
50,000 |
25 |
Launch phase; early adoption in niche markets; limited awareness |
| 2024 |
150,000 |
75 |
Expanded approvals; strategic marketing; early competitive positioning |
| 2025 |
300,000 |
150 |
Increased clinician adoption; new indications; market expansion |
| 2026 |
600,000 |
300 |
Broader global penetration; reimbursement uptake; brand recognition |
| 2027 |
1,200,000 |
600 |
Market dominance in niche segments; expanding into new indications |
| 2028 |
2,500,000 |
1,250 |
Achieving widespread acceptance; potential premium pricing |
Note: These projections are speculative but grounded in market growth trends, comparable product launches, and technological adoption rates.
Strategic Growth Opportunities
- Expansion into Male and Pediatric Markets: Potential for hormone delivery innovations.
- Combination Therapies: Partnering with biotech firms to develop multi-purpose patches.
- Regional Diversification: Focusing on Asia-Pacific, where aesthetic and reproductive health markets are expanding rapidly.
- Regulatory Milestones: Fast-track and accelerated approvals could significantly boost sales.
Risk Considerations
- Regulatory Setbacks: Delays or denials could hamper growth.
- Market Resistance: Skepticism toward new delivery systems may slow adoption.
- Competitive Intensity: Entrenched brands may accelerate innovation or reduce prices.
- Manufacturing & Supply Chain: Challenges might impact scalability.
Key Takeaways
- Market Trends Favor Innovation: Rising demand for needle-free, minimally invasive therapies positions VIVELLE-DOT favorably.
- Differentiation is Crucial: Its unique microneedle platform offers competitive advantages that can carve a niche in aesthetics and reproductive health.
- Regulatory and Education Strategy Needed: To accelerate adoption, Merz must prioritize streamlined approval pathways and clinician training.
- Sales Growth Is Promising: With strategic market entry, sales could reach USD 1.25 billion by 2028, contingent on successful regulatory navigation and market acceptance.
- Partnerships and Regional Expansion: Critical for scaling, especially in fast-growing Asian economies.
FAQs
1. What distinguishes VIVELLE-DOT from traditional dermal fillers and contraceptive methods?
VIVELLE-DOT uses a dissolvable microneedle patch for delivery, offering a needle-free, pain-free alternative with rapid absorption, reducing injection-associated discomfort common with traditional fillers or injections like Depo-Provera.
2. How does VIVELLE-DOT's market potential compare to established competitors?
While established players have extensive brand recognition, VIVELLE-DOT’s innovative delivery system and dual-purpose applications could enable it to capture niche markets within aesthetics and reproductive health, especially where patient comfort and convenience are prioritized.
3. What regulatory challenges might VIVELLE-DOT face?
As a combination of hormone therapy and cosmetic device, approval processes are complex, requiring robust safety and efficacy data. Navigating diverse regional regulatory frameworks may extend timelines but is offset by the high unmet needs.
4. What are the key factors influencing sales growth for VIVELLE-DOT?
Primarily, regulatory approvals, clinician acceptance, insurance reimbursement policies, market awareness campaigns, and regional expansion efforts will influence sales trajectory.
5. Which markets present the greatest opportunities for VIVELLE-DOT’s expansion?
Emerging markets in Asia-Pacific and Latin America, driven by increasing aesthetic procedures and women’s health initiatives, offer fertile ground for rapid growth, alongside mature markets in North America and Europe.
Conclusion
VIVELLE-DOT embodies a convergence of technological innovation and market demand across aesthetic medicine and reproductive health. The strategic deployment of its microneedle platform can open new avenues for minimally invasive, patient-centric therapies. While regulatory and competitive challenges exist, proactive positioning, robust clinical data, and targeted expansion strategies could propel annual sales beyond USD 1 billion within five years, establishing the drug as a transformative product in its domains.
References
[1] MarketsandMarkets. Aesthetic Medicine Market by Type, Application, and Region — Global Forecast to 2027.
[2] Fortune Business Insights. Global Reproductive Health Market Size, Share & Industry Analysis, 2022.