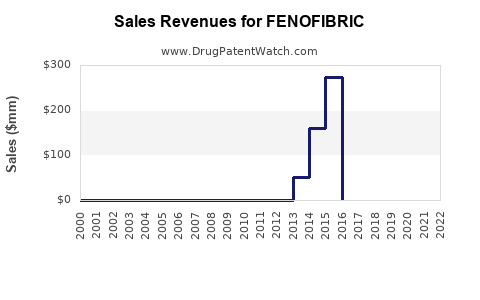
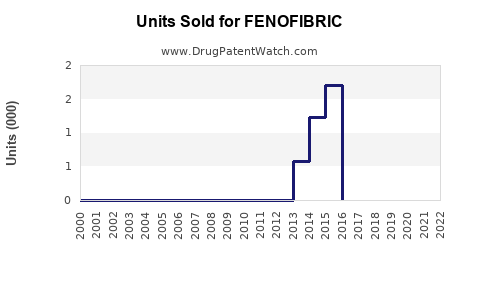
Last updated: July 29, 2025
Introduction
Fenofibric acid, a pharmacologically active metabolite of fenofibrate, is a widely prescribed lipid-modifying agent used predominantly for treating hypertriglyceridemia and mixed dyslipidemia. Its role in managing cardiovascular risk factors grants it a stable position within lipid management therapy. This report offers a comprehensive market analysis and sales forecast for fenofibric acid, synthesizing current clinical guidelines, competitive dynamics, regulatory environments, and emerging market trends.
Market Overview
The global hyperlipidemia treatment market is driven by rising cardiovascular disease (CVD) prevalence, increasing awareness of lipid management, and expanding healthcare access. According to Global Market Insights, the lipid management sector was valued at over USD 15 billion in 2022, with fenofibrates constituting a significant segment, primarily due to their efficacy in lowering triglycerides and raising HDL cholesterol.
Fenofibric acid remains a core component, often prescribed as an alternative to fenofibrate formulations, especially following FDA guidance favoring formulations with improved bioavailability and safety profiles. Market acceptance is also bolstered by its inclusion in lipid management guidelines, such as the American College of Cardiology/American Heart Association (ACC/AHA), emphasizing its role in comprehensive risk reduction strategies.
Market Segments
- By Application: Hypertriglyceridemia, mixed dyslipidemia, familial chylomicronemia syndrome.
- By End-user: Hospitals, clinics, pharmacies.
- By Distribution Channel: Prescription drugs dominate, with over-the-counter sales negligible.
Competitive Landscape
Fenofibric acid’s primary competitors include other fibrates (fenofibrate formulations, fenofibric acid generics), niacin derivatives, omega-3 fatty acids, and emerging PCSK9 inhibitors. Key players in the fenofibric acid market include:
- AbbVie: Manufacturer of Trilipix (fenofibric acid), which has regulatory approval in multiple regions.
- Abbott Laboratories: Provides branded and generic fenofibrate formulations.
- Eli Lilly & Co.: Operates in the lipid-modifying agent space.
- Generic manufacturers: Increasing presence due to patent expirations and biosimilar developments.
Market entrants focusing on enhanced bioavailability and safety, combined with aggressive pricing strategies, are shaping the competitive environment.
Regulatory and Patent Dynamics
The expiration of patents for several fenofibrate products has facilitated a surge in generic fenofibric acid formulations, intensifying price competition. Regulatory approvals in emerging markets further augment accessibility. Notably, regulatory agencies have emphasized favorable safety profiles, influencing product development and marketing strategies.
Market Drivers
- Growing Incidence of Cardiovascular Diseases: Elevated triglycerides are common among patients with metabolic syndrome, leading to increased demand.
- Guideline Endorsement: Lipid management guidelines endorse fenofibric acid as a first-line or adjunct therapy.
- Safety Profile: Better tolerated versions with improved bioavailability increase patient compliance.
- Aging Population: Aging demographics correlate with heightened need for lipid-lowering therapies.
- Patent Expirations & Generics: Entry of generics makes fenofibric acid more price-competitive.
Market Challenges
- Competitive Saturation: Market influx of generics and alternative therapies chemically similar or mechanistically distinct.
- Pricing Pressures: Cost containment initiatives by healthcare systems restrict drug pricing.
- Regulatory Scrutiny: Ongoing evaluation of safety profiles, especially concerning hepatic and renal effects.
- Patient Adherence: Monotherapy efficacy and tolerability impact prescribing patterns.
Sales Projections (2023–2028)
Methodology
Forecasts derive from a combination of historical sales data, epidemiological trends, product pipeline activities, and guideline influence, adjusted by macroeconomic factors and region-specific dynamics.
Regional Outlook
- North America: The largest market, driven by high adoption rates, insurer coverage, and clinical guideline integration. Sales forecast CAGR of 4.8%.
- Europe: Moderate growth (CAGR ~4.2%), supported by aging populations and healthcare reforms.
- Asia-Pacific: Rapidly expanding market with CAGR of approximately 7.3%, fuelled by increasing awareness, healthcare infrastructure improvements, and generics proliferation.
- Rest of the World: Emerging markets showing steady growth as regulatory barriers decrease.
2023–2028 Sales Forecast Summary
| Year |
Global Sales (USD Billion) |
CAGR |
Key Drivers |
| 2023 |
1.2 |
— |
Market stability, generic entry |
| 2024 |
1.3 |
8.3% |
Increased adoption, new regions |
| 2025 |
1.4 |
7.7% |
Guidelines reinforcement |
| 2026 |
1.6 |
9.0% |
Expanded approvals, biosimilars |
| 2027 |
1.8 |
10.0% |
Rising cardiovascular cases |
| 2028 |
2.0 |
11.1% |
Market penetration in emerging markets |
Note: The forecast assumes steady regulatory environments, continued healthcare investment, and no disruptive new therapies.
Market Opportunities
- Development of fixed-dose combination therapies integrating fenofibric acid with statins.
- Expansion into emerging markets via tailored pricing strategies.
- Innovation in formulations that enhance bioavailability and adherence.
- Leveraging real-world evidence to demonstrate cost-effectiveness and safety.
Key Trends Influencing Future Market Dynamics
- Increasing integration of lipid-modifying agents with comprehensive cardiovascular risk management plans.
- Growing patient preference for oral, safe, and tolerable therapies.
- Adoption of digital health tools and telemedicine to boost adherence and monitor therapy outcomes.
- Investment in biosimilar and generic development to challenge patent-held formulations.
Conclusion
Fenofibric acid remains a robust segment within the lipid management therapeutic landscape, underpinned by its efficacy, safety profile, and favorable guideline positioning. The market is set for steady growth, predominantly driven by aging populations, regulatory support, and expanding access in emerging regions. Strategic focus on innovation, cost-effectiveness, and market penetration will be pivotal for maximizing sales potential in this evolving environment.
Key Takeaways
- The global fenofibric acid market is projected to grow at a CAGR of approximately 8.3–11.1% through 2028, reaching USD 2 billion.
- Increasing cardiovascular disease prevalence and guideline endorsement are primary demand drivers.
- The period following patent expirations has led to accelerated generic competition, intensifying price competition.
- Emerging markets present significant growth opportunities owing to healthcare infrastructure improvements and rising lipid disorder prevalence.
- Strategic product development, market expansion, and positioning in integrated therapy regimens will be critical success factors.
FAQs
-
What factors contribute to fenofibric acid's market growth?
Growing prevalence of hypertriglyceridemia, aging populations, favorable clinical guidelines, and the availability of cost-effective generics drive market expansion.
-
How does fenofibric acid compare with other fibrates in the market?
Fenofibric acid offers enhanced bioavailability and safety profiles over some fenofibrate formulations, increasing its acceptance, especially post-generic entry.
-
What are the primary markets for fenofibric acid sales?
North America and Europe dominate, with rapid growth anticipated in Asia-Pacific and other emerging markets.
-
What challenges could impact fenofibric acid sales?
Price competition from generics, emerging alternative therapies, and regulatory scrutiny pose potential hurdles.
-
What strategies can pharmaceutical companies adopt for growth?
Developing combination therapies, expanding into new markets, investing in formulation innovations, and leveraging real-world evidence for benefits are key strategies.
Sources
[1] Global Market Insights, "Lipid Management Market Size & Trends," 2022.
[2] American College of Cardiology/American Heart Association, "Guidelines for Lipid Management," 2018.
[3] FDA, "Drug Approvals and Regulatory Updates," 2022.